Neutrophil-Mimetic oleanolic acid-loaded Liposomes targeted to alleviate oxidative stress for renal ischemia-reperfusion injury treatment
- PMID: 40529379
- PMCID: PMC12173123
- DOI: 10.1016/j.ijpx.2025.100344
Neutrophil-Mimetic oleanolic acid-loaded Liposomes targeted to alleviate oxidative stress for renal ischemia-reperfusion injury treatment
Abstract
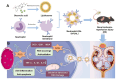
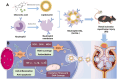
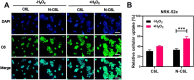
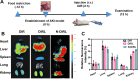
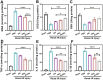
Acute kidney injury (AKI) is a prevalent clinical condition characterized by a sudden decline or loss of renal function, exacerbated by the lack of effective diagnostic and therapeutic tools. Renal ischemia-reperfusion injury serves as the primary cause of AKI, initiating a complex signaling cascade that mediates renal cell necrosis, apoptosis, and inflammation. Oxidative stress plays a crucial role in the pathogenesis and progression of ischemia-reperfusion injury, thus prompting the exploration of antioxidants as potential therapeutic interventions. Oleanolic acid, derived from natural plant extracts, exhibits significant antioxidant and anti-inflammatory properties; however, its clinical application has been hindered by inherent limitations such as poor water solubility and low bioavailability. To address this issue, we developed an innovative approach involving oleanolic acid-loaded liposomes fused with neutrophil membranes, resulting in hybrid liposomes (N-OAL). This strategy aims to enhance the accumulation and retention of N-OAL at inflammatory sites associated with AKI through biomimetic chemotaxis mediated by neutrophil membranes specifically targeting damaged renal tubular epithelial cells. The optimized N-OAL presented a spherical morphology with an average particle size of 125.6 ± 4.9 nm and a surface potential of -4.8 ± 0.3 mV. In addition, N-OAL exhibited favorable sustained release, outstanding stability, and satisfactory biocompatibility. In vitro studies demonstrated that N-OAL effectively attenuated H2O2-induced intracellular reactive oxygen species generation and inflammation while exhibiting superior antioxidant and anti-apoptotic properties. Furthermore, our in vivo results confirmed the remarkable protective effect of N-OAL on oxidative-damaged renal tissue caused by AKI induction. Overall, our study provides novel insights into targeted delivery strategies for oleanolic acid therapy in acute kidney injury.
Keywords: Ischemia/reperfusion injury; Liposome; Neutrophil-mimetic; Oleanolic acid; Oxidative stress.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Castaneda M.P., Swiatecka-Urban A., Mitsnefes M.M., Feuerstein D., Kaskel F.J., Tellis V., Devarajan P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation. 2003;76:50–54. - PubMed
-
- Chen S., Li Q., Shi H., Li F., Duan Y., Guo Q. New insights into the role of mitochondrial dynamics in oxidative stress-induced diseases. Biomed. Pharmacother. 2024;178 - PubMed
-
- Chen Y., Zhu M., Huang B., Jiang Y., Su J. Advances in cell membrane-coated nanoparticles and their applications for bone therapy. Biomaterials Advances. 2023;144 - PubMed
LinkOut - more resources
Full Text Sources

