Automated segmentation of target volumes in breast cancer radiotherapy, impact on target size and dose to organs at risk
- PMID: 40529410
- PMCID: PMC12173629
- DOI: 10.1016/j.ctro.2025.100986
Automated segmentation of target volumes in breast cancer radiotherapy, impact on target size and dose to organs at risk
Abstract
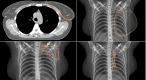
Introduction: Target volume delineation is crucial in breast cancer radiotherapy planning but involves significant interobserver variability. Deep learning (DL) models may reduce this variability, saving time and costs. However, current DL-models do not consider clinical data, such as tumor location and patient comorbidity, to adjust the target and reduce dose to organs at risk (OAR). This study compares clinically defined target volumes to those generated by a DL-model in terms of size, geometric overlap, and dose to OAR.
Method: For a hypothetical breast cancer patient, we compared target volumes constructed by Swedish radiotherapy clinics and two DL-models, Raystation and MVision. Geometrical overlap was evaluated, as well as the impact of differences in target delineation on dose to OAR. Treatment plans for locoregional vs. breast-only 3D-conformal radiotherapy were generated.
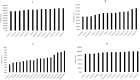
Results: CTV-structures for the breast, lymph nodes level I-IV, and internal mammary nodes were available for 10, 11, and 14 centers respectively. Volume of the CTV-breasts varied between 770-890cc, and the total CTV-volumes (breast + lymph nodes) between 875-1003cc. The DL-models did not constitute the largest nor smallest breast or total CTV-volumes, and geometric overlap between structures was relatively good. Evaluating dose to OAR from dose plans based on the respective CTV-volumes for locoregional radiotherapy, this was comparable between the DL-models and the mean of the CTVs generated by the clinics. In radiotherapy of only the breast, the CTV-breasts constructed by the DL-models gave the highest heart doses due to their proximity to the chest wall, affecting field angle choices. No difference was seen in dose to the ipsilateral lung, thyroid gland, or humeral head.
Conclusion: DL-models for target delineation have great potential. However, their introduction must be closely monitored since even small differences compared to clinical standards may affect doses to OAR in 3D conformal breast cancer radiotherapy.
Keywords: AI contouring; Breast cancer; Deep learning segmentation; Dosimetric data; Radiotherapy; Target volume delineation.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Dipasquale G., et al. Automatic segmentation of breast in prone position: Correlation of similarity indexes and breast pendulousness with dose/volume parameters. Radiother Oncol. 2016;120(1):124–127. - PubMed
-
- Buhl E.S., et al. Population based audit of heart radiation doses in 6925 high-risk breast cancer patients from the Danish breast cancer group RT Nation study. Radiother Oncol. 2025;202 - PubMed
LinkOut - more resources
Full Text Sources

