Assessing the efficacy of the natural disaccharide trehalose in ameliorating diet-induced obesity and metabolic dysfunction
- PMID: 40529415
- PMCID: PMC12171462
- DOI: 10.3389/fnut.2025.1580684
Assessing the efficacy of the natural disaccharide trehalose in ameliorating diet-induced obesity and metabolic dysfunction
Abstract
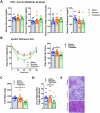
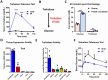
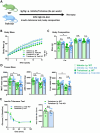
Trehalose is a naturally occurring disaccharide with versatile commercial applications and health benefits, including promise as a therapeutic for obesity and diabetes. Although numerous previous reports purport the therapeutic uses of orally ingested trehalose, the abundance of glycosidases in the gastrointestinal tract suggest the potential for significant limitations of oral trehalose that have not been addressed. We first fed mice a high-fat diet (HFD) while providing trehalose by both oral and intraperitoneal routes. This combined strategy was broadly efficacious in reversing HFD-induced weight gain, fat mass, insulin resistance, and the development of hepatosteatosis. In contrast, oral-only trehalose failed to improve HFD-induced obesity and insulin resistance. This was due to trehalase (Treh)-mediated metabolism as blood trehalose levels remained low despite a significant rise in glucose. We next developed systemically deficient Trehalase (Treh-KO) mice to enhance the efficacy of trehalose. Surprisingly, oral trehalose therapy could not be facilitated resulting in neither an increase in serum trehalose levels nor metabolic benefits. Parenteral trehalose resulted in higher trehalose levels with lower serum glucose in Treh-KO mice, yet no additive metabolic benefits were observed. Overall, our findings still support a therapeutic role for trehalose in obesity and metabolic disease but with practical limitations in its delivery by oral route.
Keywords: hepatosteatosis; insulin resistance; obesity; oral vs. parenteral; trehalase; trehalose.
Copyright © 2025 Yeh, Evans, Jeong, Liu, Ajam, Cosme, Huang, Peroumal, Zhang, Javaheri, Cho, Lodhi and Razani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

