Catechin inhibits ox-LDL-induced ferroptosis in vascular smooth muscle cells to alleviate and stabilize atherosclerosis
- PMID: 40529424
- PMCID: PMC12171153
- DOI: 10.3389/fnut.2025.1594708
Catechin inhibits ox-LDL-induced ferroptosis in vascular smooth muscle cells to alleviate and stabilize atherosclerosis
Abstract
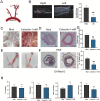
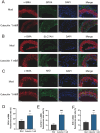
Atherosclerosis (AS) is a chronic, progressive vascular disease marked by lipid deposition in the arterial intima, vascular wall thickening, luminal narrowing, and compromised blood flow. Although macrophage-derived foam cells are well-studied, vascular smooth muscle cells (VSMCs) also substantially contribute to AS, particularly when they transition into foam cells under oxidative stress. Accumulating evidence suggests that ferroptosis-an iron-dependent, regulated cell death mechanism characterized by lipid peroxidation-exacerbates AS pathology through oxidative damage and vascular dysfunction. Catechin, a potent antioxidant abundant in green tea, has demonstrated efficacy in reducing oxidative stress; however, its role in suppressing VSMC ferroptosis induced by oxidized low-density lipoprotein (ox-LDL) remains unclear. Here, we evaluated catechin's capacity to protect VSMCs against ox-LDL-induced ferroptosis, focusing on its modulation of the Nrf2/SLC7A11/GPX4 axis. Mouse vascular smooth muscle (MOVAS) cells were incubated with ox-LDL to induce foam cell formation and ferroptosis. We assessed intracellular iron, lipid peroxidation, reactive oxygen species (ROS), and antioxidant defenses and examined mitochondrial ultrastructure via transmission electron microscopy (TEM). Ferroptosis-related proteins were measured by Western blot, immunofluorescence, and qPCR. In vivo, ApoE-/- mice on a high-fat diet (HFD) underwent partial carotid ligation with local catechin administration to investigate plaque formation and ferroptosis in arterial tissue. Our results show that catechin reduced intracellular Fe2+, decreased ROS and malondialdehyde (MDA) levels, and preserved mitochondrial integrity in ox-LDL-exposed MOVAS cells. Catechin also enhanced GSH and SOD levels and restored GPX4, SLC7A11, and Nrf2 expression, thereby reducing foam cell formation. In ApoE-/- mice, catechin reduced plaque size, mitigated lipid deposition, and upregulated GPX4, SLC7A11, and Nrf2 in the arterial wall. Collectively, these findings confirm that catechin prevents ox-LDL-induced ferroptosis in VSMCs by activating the Nrf2/SLC7A11/GPX4 pathway, highlighting its potential therapeutic value for atherosclerosis. This study provides additional evidence for the role of dietary polyphenols in regulating ferroptosis within VSMCs.
Keywords: GPX4; Nrf2 pathway; atherosclerosis; catechin; ferroptosis; ox-LDL; oxidative stress; vascular smooth muscle cells.
Copyright © 2025 Guo, Xie, Yuan, Liao and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Paclitaxel Attenuates Atherosclerosis by Suppressing Macrophage Ferroptosis and Improving Lipid Metabolism via the Sirt1/Nrf2/GPX4 Pathway.FASEB J. 2025 Aug 15;39(15):e70917. doi: 10.1096/fj.202501047RR. FASEB J. 2025. PMID: 40779351
-
Folic Acid Ameliorates Neuronal Ferroptosis in Aging by Up-Regulating SLC7A11-GSH-GPX4 Antioxidant Pathway and Increasing Cystine Levels.Int J Mol Sci. 2025 Jul 11;26(14):6669. doi: 10.3390/ijms26146669. Int J Mol Sci. 2025. PMID: 40724917 Free PMC article.
-
Huotan Jiedu Tongluo Decoction targets Nrf2-mediated macrophage autophagy to inhibit ferroptosis and reduce atherosclerotic lesions.Phytomedicine. 2025 Sep;145:157012. doi: 10.1016/j.phymed.2025.157012. Epub 2025 Jun 23. Phytomedicine. 2025. PMID: 40609383
-
Ferroptosis: a potential therapeutic target in cardio-cerebrovascular diseases.Mol Cell Biochem. 2025 Jul;480(7):4379-4399. doi: 10.1007/s11010-025-05262-7. Epub 2025 Mar 27. Mol Cell Biochem. 2025. PMID: 40148662 Review.
-
Ferroptosis and Nrf2 Signaling in Head and Neck Cancer: Resistance Mechanisms and Therapeutic Prospects.Antioxidants (Basel). 2025 Aug 13;14(8):993. doi: 10.3390/antiox14080993. Antioxidants (Basel). 2025. PMID: 40867889 Free PMC article. Review.
References
-
- Alencar GF, Owsiany KM, Karnewar S, Sukhavasi K, Mocci G, Nguyen AT, et al. Stem cell pluripotency genes Klf4 and Oct4 regulate complex SMC phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation. (2020) 142:2045–59. 10.1161/CIRCULATIONAHA.120.046672 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

