Developmental mechanisms underlying pediatric epilepsy
- PMID: 40529445
- PMCID: PMC12172511
- DOI: 10.3389/fneur.2025.1586947
Developmental mechanisms underlying pediatric epilepsy
Abstract
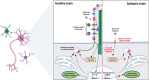
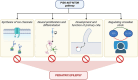
Pediatric epilepsy affects a large proportion of children, with a huge variability in seizure onset. Due to complicated etiology, wide range of associated comorbidities, and difficulty in obtaining clear physiological data from children, epilepsy management in pediatric patients often poses a critical challenge. Importantly, around 30% of these patients remain non-responsive to current anti-seizure drugs and develop a higher risk of developmental and cognitive delay and, in worse situations, premature death. One of the key treatment methods currently used for drug-resistant epilepsies is surgical resection of the epileptic foci. However, such patients often develop new epileptic foci post-surgery. This, in turn, enhances the need for recurrent invasive brain surgeries, impairing the overall quality of life in these children. Thus, mechanistic understanding of different types of pediatric epilepsy is critical to discovering more targeted molecular approach(es). For a long time, the occurrence of epilepsy was considered solely due to the abnormal functioning of single ion channels. However, in recent years, a huge number of genetic and non-genetic (environmental) factors have been associated with different types of pediatric epilepsy. Clinical diagnoses, coupled with a basic understanding of molecular and cellular mechanisms using different model systems, have been instrumental in unraveling new avenues for modern non-invasive targeted pharmacological therapies. Yet, the field has just started to evolve, and many challenges and contradictory hypotheses still exist. This comprehensive review discusses underlying developmental mechanisms associated with pediatric epilepsy. Specifically, we highlight how the PI3K-AKT-MTOR pathway acts as a critical node interconnecting the diverse mechanistic strategies, that may eventually help overcome the seizure burden in the future.
Keywords: Cilia; PI3K-AKT–MTOR pathway; SUDEP; drug resistance; epilepsy – abnormalities; neurodevelopment; pediatric epilepsy; sleep and circadian rhythm.
Copyright © 2025 Lolam and Roy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670, PMID: - DOI - PubMed
-
- Pressler RM, Cilio MR, Mizrahi EM, Moshé SL, Nunes ML, Plouin P, et al. The ILAE classification of seizures and the epilepsies: Modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia. (2021) 62:615–28. doi: 10.1111/epi.16815, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

