Treatment of indolent systemic mastocytosis with sarilumab is not supported in a randomized trial
- PMID: 40529483
- PMCID: PMC12172267
- DOI: 10.1016/j.jacig.2025.100498
Treatment of indolent systemic mastocytosis with sarilumab is not supported in a randomized trial
Abstract
Background: Indolent systemic mastocytosis is a clonal mast cell disease that results in an increase in mast cells in the skin, bone marrow, and other organ systems. IL-6 has been shown to promote mast cell maturation, proliferation, and reactivity in vitro. Serum levels of IL-6 correlate with severity of disease and risk of progression of systemic disease.
Objective: We conducted a double-blind placebo-controlled study to assess safety and efficacy for the use of sarilumab in improving the quality of life for those with indolent systemic mastocytosis. ClinicalTrial.gov registration NCT03770273.
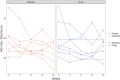
Methods: A double-blind trial randomized 16 participants. The primary analysis compared the arms on the Mastocytosis Quality of Life Questionnaire (MC-QoL) index at 16 weeks, adjusting for the baseline MC-QoL. Mean baseline MC-QoL was 47.8.
Results: The difference between the arms in the primary analysis was not statistically significant, with the results favoring the placebo arm; mean absolute MC-QoL improvement in the placebo arm was 14.7 relative to 8.9 in the sarilumab arm. The estimated treatment effect from regression analysis was a 6.0-unit advantage of MC-QoL in the placebo arm (P = .40; 95% confidence interval, -8.9, 20.8), limiting a possible sarilumab advantage to at most 9 units of MC-QoL. Similar conclusions were observed for other quality-of-life indices.
Conclusions: Results in this small trial did not support using sarilumab to treat this population and highlights the importance of double-blind randomized studies.
Keywords: Mastocytosis; anti–IL-6; mast cells; sarilumab; tryptase.
Conflict of interest statement
Supported by the 10.13039/100006492Division of Intramural Research, National Institute of Allergy and Infectious Diseases, 10.13039/100000002National Institutes of Health (NIH). This project was funded in whole or in part with federal funds from the 10.13039/100000054National Cancer Institute, NIH, under contract 75N91019D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services; nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Figures
References
-
- Horny H.P., Metcalfe D.D., Akin C., Bennett J.M., Arber D.A., Bain B.J., et al. In: WHO classification of tumours of haematopoietic and lymphoid tissues. World Health Organization. 4th ed. Swerdlow S.H., editor. International Agency for Research on Cancer; Lyon (France): 2008. Mastocytosis; pp. 62–69.
-
- Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. - PubMed
-
- Mihara M., Hashizume M., Yoshida H., Suzuki M., Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–159. - PubMed
-
- Brockow K., Akin C., Huber M., Metcalfe D.D. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol. 2005;115:216–223. - PubMed
-
- Fujii K., Ohgou N. Up-regulation of IL-6 mRNA in peripheral blood mononuclear cells of patients with acute urticaria. J Dermatol. 2004;31:242–243. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical