Trapidil attenuates diabetic cardiomyopathy via GPX3/Nrf2-mediated inhibition of myocardial pyroptosis
- PMID: 40529489
- PMCID: PMC12170559
- DOI: 10.3389/fphar.2025.1566622
Trapidil attenuates diabetic cardiomyopathy via GPX3/Nrf2-mediated inhibition of myocardial pyroptosis
Abstract
Background: Currently, there is a paucity of clinically effective medications for the treatment of diabetic cardiomyopathy (DCM), while the strategy of drug repurposing offers a promising avenue for advancing therapeutic development.
Methods: The investigation explored the ameliorative effects and uncovered underlying mechanisms of trapidil (TRA), a drug commonly employed in the management of coronary heart disease, on DCM by inhibiting myocardial pyroptosis. Type 1 DCM models were established utilizing C57BL/6 mice and primary neonatal mouse cardiomyocytes (NMCMs), which were subsequently treated with TRA.
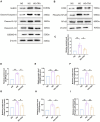
Results: Results demonstrated that in DCM mice, TRA significantly enhanced cardiac function, effectively alleviated pathological changes in myocardial tissue, reversed ultrastructural alterations, and reduced pyroptosome formation in myocardial cells. TRA significantly increased the body weight of the mice in the DCM model group, whereas there was no significant alteration in blood glucose levels following TRA treatment. In the myocardial tissue of DCM mice and high-glucose (HG)-treated NMCMs, TRA was found to correct the aberrant expression of key proteins involved in pyroptosis, including cleaved-caspase1, NLRP3, phospho-NF-κB cyclooxygenase-2, interleukin Cleaved-IL-1β, Cleaved-IL-18, and gasdermin D. Furthermore, TRA effectively curtailed the excessive production of ROS and augmented the mitochondrial membrane potential in NMCMs under the HG environment. Proteomics analysis identified 90 differentially expressed proteins between DCM mice and TRA-treated mice, with glutathione peroxidase 3 (GPX3) emerging as a standout due to its critical role in the cellular antioxidant defense system. Further investigations revealed that the protein and mRNA levels of GPX3, as well as the activated Nrf2 protein levels, were significantly downregulated in the myocardial tissue of DCM mice and HG-treated NMCMs cells. However, these levels were notably upregulated following TRA treatment. Upon knocking down GPX3 mRNA expression using siRNA technology, the anti-pyroptotic effect of TRA in cardiomyocytes was markedly diminished, and the level of activated Nrf2 protein also significantly decreased.
Conclusion: In conclusion, TRA holds potential for improving DCM, with the inhibition of myocardial pyroptosis via the GPX3/Nrf2 pathway playing a pivotal role. HG-induced Downregulation of the GPX3/Nrf2 pathway is a critical mechanism underlying pyroptosis in DCM. This pathway can be targeted for the design of DCM-related therapeutics, utilizing the aforementioned signaling mechanisms.
Keywords: GPX3; Nrf2; diabetic cardiomyopathy; pyroptosis; trapidil.
Copyright © 2025 Wang, Sun, Wang, Xu, Wang, Zhang, Song, Wang and Qi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
piR112710 attenuates diabetic cardiomyopathy through inhibiting Txnip/NLRP3-mediated pyroptosis in db/db mice.Cell Signal. 2024 Oct;122:111333. doi: 10.1016/j.cellsig.2024.111333. Epub 2024 Aug 3. Cell Signal. 2024. PMID: 39102928
-
Vitamin D improves diabetic cardiomyopathy by inhibiting pyroptosis through the NOX4/NLRP3 inflammasome pathway.Food Funct. 2025 Aug 11;16(16):6718-6732. doi: 10.1039/d5fo00717h. Food Funct. 2025. PMID: 40748236
-
Mitochondrial ultrastructural pathology in diabetic cardiomyopathy: integrated analysis via scanning electron microscopy and 3D visualization imaging.Cardiovasc Diabetol. 2025 Aug 13;24(1):331. doi: 10.1186/s12933-025-02884-5. Cardiovasc Diabetol. 2025. PMID: 40804395 Free PMC article.
-
New Insights on the Potential Role of Pyroptosis in Parkinson's Neuropathology and Therapeutic Targeting of NLRP3 Inflammasome with Recent Advances in Nanoparticle-Based miRNA Therapeutics.Mol Neurobiol. 2025 Jul;62(7):9365-9384. doi: 10.1007/s12035-025-04818-4. Epub 2025 Mar 18. Mol Neurobiol. 2025. PMID: 40100493 Review.
-
The role of Nrf2 signaling pathway in diabetic cardiomyopathy: from pathogenesis to traditional Chinese medicine interventions.Front Cardiovasc Med. 2025 Jul 14;12:1492499. doi: 10.3389/fcvm.2025.1492499. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40727576 Free PMC article. Review.
References
-
- Burk R. F., Olson G. E., Winfrey V. P., Hill K. E., Yin D. (2011). Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am. J. physiology. Gastrointest. liver physiology 301 (1), G32–G38. 10.1152/ajpgi.00064.2011 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

