Blood metabolites as mediators in erectile dysfunction: insights from a multi-center proteomics and genetic study
- PMID: 40529500
- PMCID: PMC12171135
- DOI: 10.3389/fphar.2025.1568780
Blood metabolites as mediators in erectile dysfunction: insights from a multi-center proteomics and genetic study
Abstract
Objective: This study aims to identify circulating proteins causally associated with erectile dysfunction (ED) using Mendelian randomization (MR) analysis.
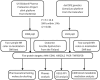
Methods: We utilized two of the largest multi-center proteomics databases as exposures and the FinnGen database as the outcome source. A large-scale two-sample MR analysis, including coloc colocalization analysis and SMR (Summary data-based Mendelian Randomization) analysis, was conducted to evaluate the reliability of proteomic effects on ED outcomes. Additionally, MR mediation analysis involving 1,400 blood metabolites was performed to investigate how these proteins mediate the effect of blood metabolites on ED. Finally, protein-protein interaction analysis, pathway enrichment analysis, druggability assessments, and molecular docking were employed to further elucidate the mechanisms of ED and identify potential therapeutic targets.
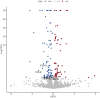
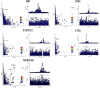
Results: Eight circulating proteins (AMN, ESM1, KIR2DL2, PIGR, SPINT1, SPP1, TNFRSF6B, TMEM9) were identified as causally associated with ED based on two-sample MR and coloc colocalization criteria. Among these, five proteins (AMN, ESM1, KIR2DL2, PIGR, TNFRSF6B) satisfied SMR validation, while SPINT1, TMEM9, and SPP1 were excluded. Several of these proteins were found to mediate the relationship between metabolites and ED. These proteins are recognized as either druggable targets or existing drug targets, with molecular docking results demonstrating favorable interactions with various drug candidates.
Conclusion: Using MR analysis, we identified five proteins associated with ED, clarified protein-mediated mechanisms, and proposed promising therapeutic targets for ED.
Keywords: blood metabolites; erectile dysfunction; mendelian randomization; precision therapy; proteomics.
Copyright © 2025 Chen, Zhao, Zhang, Zhu, Zuo, Nie, Fu, Wang, Tang and Fu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Exploring potential therapeutic targets for cardiomyopathy: A proteome-wide Mendelian randomization analysis.Medicine (Baltimore). 2025 Jun 13;104(24):e42681. doi: 10.1097/MD.0000000000042681. Medicine (Baltimore). 2025. PMID: 40527822 Free PMC article.
-
IGF1R as a protective target in antidiabetic drug-mediated prevention of erectile dysfunction: Insights from genetics.Reprod Toxicol. 2025 Jul 12;137:108999. doi: 10.1016/j.reprotox.2025.108999. Online ahead of print. Reprod Toxicol. 2025. PMID: 40659136
-
Interconnections between BDH1-plasma protein-type 2 diabetes Mellitus: a mediated mendelian randomization analysis using plasma proteomics.Sci Rep. 2025 Jan 27;15(1):3342. doi: 10.1038/s41598-025-88196-w. Sci Rep. 2025. PMID: 39870808 Free PMC article.
-
Two-Sample Network Mendelian Randomization and Single-Cell Analysis Reveal the Causal Associations and Underlying Mechanisms Between Antihypertensive Drugs and Kidney Cancer.J Cancer. 2025 Jun 12;16(8):2690-2705. doi: 10.7150/jca.110850. eCollection 2025. J Cancer. 2025. PMID: 40535814 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Anaokar S., Kodali R., Jonik B., Renne M. F., Brouwers J. F. H. M., Lager I., et al. (2019). The glycerophosphocholine acyltransferase Gpc1 is part of a phosphatidylcholine (PC)-remodeling pathway that alters PC species in yeast. J. Biol. Chem. 294 (4), 1189–1201. 10.1074/jbc.RA118.005232 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

