Synthesis of mangiferin derivatives, complexes, and carriers as potential therapeutic candidates for cancer treatment: an update
- PMID: 40529503
- PMCID: PMC12170590
- DOI: 10.3389/fphar.2025.1598719
Synthesis of mangiferin derivatives, complexes, and carriers as potential therapeutic candidates for cancer treatment: an update
Abstract
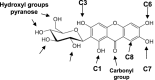
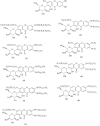
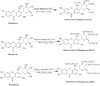
Mangiferin (MF), a xanthonoid polyphenol, its derivatives, coordination and inclusion complexes, and carriers have demonstrated notable antitumor activity in vitro and in vivo. However, their clinical application remains limited due to MF's poor water solubility and low systemic bioavailability. This review critically summarizes advances in the synthesis of MF derivatives and formulation strategies, such as metal complexes, cyclodextrin inclusion systems, and nanocarriers, developed over the past decade to enhance MF's bioavailability and therapeutic efficacy. Promising results include glycosylated derivatives, MF-Se (IV) metal complexes, and β-cyclodextrin complexes, each contributing to improved solubility and cytotoxicity profiles. Continued research is essential to bridge the gap between experimental success and its clinical implementation in cancer therapy.
Keywords: antitumor effect; bioavailability; mangiferin; mangiferin carriers; mangiferin complexes; mangiferin derivatives.
Copyright © 2025 Melo-Betances, Rodríguez-Bautista and Núñez-Sellés.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
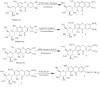
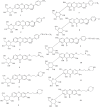
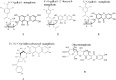
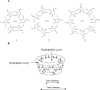

References
-
- Aboyewa J. A., Sibuyi N. R., Goboza M., Murtz L. A., Oguntibeju O. O., Meyer M. (2022). Co-treatment of Caco-2 cells with doxorubicin and gold nanoparticles produced from cyclopia intermedia extracts or mangiferin enhances drug effects. Nanomaterials 12 (21), 3918. 10.3390/nano12213918 - DOI - PMC - PubMed
-
- Ahmad N., Khan M. F., Ullah Z., Chaudhary A. A., Alawam A. S., Khalid M. S., et al. (2024). Development and evaluation of polysorbate-80 coated Mangiferin PLGA nanoparticles used in the treatment of cerebral ischemia. Polym. Bull. 81 (8), 7035–7069. 10.1007/s00289-023-05030-x - DOI
-
- Al-Yasiri A. Y., Khoobchandani M., Cutler C. S., Watkinson L., Carmack T., Smith C. J., et al. (2017). Mangiferin functionalized radioactive gold nanoparticles (MGF-198AuNPs) in prostate tumor therapy: green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalt Trans. 46, 14561–14571. 10.1039/C7DT00383H - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

