Exosomal miR-24-3p mediates myoblast-macrophage crosstalk to promote abdominal muscle repair
- PMID: 40529508
- PMCID: PMC12170637
- DOI: 10.3389/fphar.2025.1604776
Exosomal miR-24-3p mediates myoblast-macrophage crosstalk to promote abdominal muscle repair
Abstract
Objective: The objective of this study was to explore the role of exosomal miR-24-3p in facilitating communication between myoblasts and macrophages, and to assess its potential in promoting abdominal muscle repair.
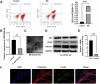
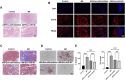
Methods: We utilized C2C12 myoblasts and RAW 264.7 macrophages, inducing the latter into an M2 phenotype. miR-24-3p levels were manipulated via transfection, and exosomes were isolated from M2 macrophages using ultracentrifugation. Exosome characterization was performed using TEM and Western blot. In vitro assays evaluated C2C12 cell proliferation, migration, and differentiation. In vivo, a cardiotoxin-induced mouse model of muscle injury was used to assess the effects of exosomal miR-24-3p on muscle repair, including histological assessment and analysis of cytokine and metabolic markers.
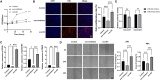
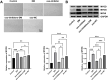
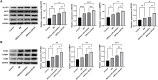
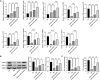
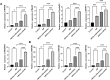
Results: Our results demonstrated that exosomal miR-24-3p, when isolated from M2 macrophages, was effectively internalized by C2C12 cells and significantly enhanced their metabolic activity, proliferation, and migratory capabilities. Moreover, it induced cellular differentiation, as observed under microscopic examination. In the abdominal muscle injury model, the administration of exosomal miR-24-3p led to a reduction in muscle fiber damage, fibrosis, and inflammation. It also promoted the restoration of glucose and lipid metabolism, which is critical for the energy demands of regenerating muscle. Furthermore, exosomal miR-24-3p upregulated the expression of genes associated with muscle cell proliferation and differentiation, suggesting its potential role in muscle repair.
Conclusion: In conclusion, exosomal miR-24-3p plays a significant role in facilitating abdominal muscle repair by mediating the interaction between myoblasts and macrophages.
Keywords: abdominal muscle repair; exosomal miR-24-3p; macrophage; muscle regeneration; myoblast.
Copyright © 2025 Liu, Zou, Cao, Zhu, Zhu and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Exosomes derived from hypoxia-preconditioned M2 macrophages alleviate degeneration in knee osteoarthritis through the miR‑124‑3p/STAT3 axis.J Transl Med. 2025 Jul 10;23(1):772. doi: 10.1186/s12967-025-06808-5. J Transl Med. 2025. PMID: 40640893 Free PMC article.
-
Hypoxic breast cancer cell-derived exosomal miR-143-3p targets RICTOR to regulate M2 macrophage polarization, thereby modulating cancer cell invasiveness.Hum Cell. 2025 May 30;38(4):114. doi: 10.1007/s13577-025-01232-9. Hum Cell. 2025. PMID: 40447873
-
BAP31 Promotes Epithelial-Mesenchymal Transition Progression Through the Exosomal miR-423-3p/Bim Axis in Colorectal Cancer.Int J Mol Sci. 2025 Jun 7;26(12):5483. doi: 10.3390/ijms26125483. Int J Mol Sci. 2025. PMID: 40564945 Free PMC article.
-
Exosomal MicroRNAs as Epigenetic Biomarkers for Endometriosis: A Systematic Review and Bioinformatics Analysis.Int J Mol Sci. 2025 May 9;26(10):4564. doi: 10.3390/ijms26104564. Int J Mol Sci. 2025. PMID: 40429709 Free PMC article. Review.
-
Decoding the Tumor Microenvironment: Exosome-Mediated Macrophage Polarization and Therapeutic Frontiers.Int J Biol Sci. 2025 Jun 20;21(9):4187-4214. doi: 10.7150/ijbs.114222. eCollection 2025. Int J Biol Sci. 2025. PMID: 40612677 Free PMC article. Review.
References
-
- Baysoy A., Tian X., Zhang F., Renauer P., Bai Z., Shi H., et al. (2024). Spatially resolved in vivo CRISPR screen sequencing via perturb-DBiT. bioRxiv, 10.1101/2024.11.18.624106 - DOI
-
- Fang S., Xu C., Zhang Y., Xue C., Yang C., Bi H., et al. (2016). Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem cells Transl. Med. 5, 1425–1439. 10.5966/sctm.2015-0367 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

