Endolysins and membrane-active peptides: innovative engineering strategies against gram-negative bacteria
- PMID: 40529583
- PMCID: PMC12170589
- DOI: 10.3389/fmicb.2025.1603380
Endolysins and membrane-active peptides: innovative engineering strategies against gram-negative bacteria
Abstract
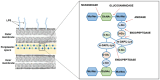
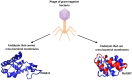
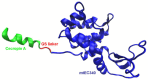
Endolysins, bacteriophage-encoded peptidoglycan hydrolases, offer promising potential in antibacterial therapy, including treatments targeting gram-negative bacteria. While these enzymes naturally act primarily on gram-positive bacteria, their application against gram-negative pathogens is more challenging due to the presence of a dual-layer cell membrane, which acts as a protective barrier. However, innovative approaches, such as fusing endolysins with antimicrobial peptides (AMPs), have demonstrated increased efficacy against gram-negative bacteria. Modifying endolysins by introducing hydrophobic properties or positive charges or combining them with agents that disrupt the outer membrane enhances their bactericidal activity. Moreover, phage endolysins that exhibit activity against gram-negative bacteria are a promising source of membrane-active peptides. Identifying new peptide sequences derived from endolysins capable of penetrating the bacterial cell membrane represents a novel and increasingly explored research direction. Studying these innovative strategies had yielded promising results, though the field remains under active investigation and development. Ongoing efforts aim to optimize these approaches to improve their effectiveness against antibiotic-resistant gram-negative bacterial strains, which are particularly difficult to treat with conventional antibiotics. This review summarizes the latest advancements and solutions in the field, highlighting the potential of endolysins and membrane-active peptides as next-generation antibacterial agents.
Keywords: antibiotics; antimicrobial agents; antimicrobial peptides; bacteriophage; endolysins; gram-negative bacteria; peptides.
Copyright © 2025 Wojciechowska.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abdelkader K., Gutiérrez D., Tamés-Caunedo H., Ruas-Madiedo P., Safaan A., Khairalla A. S., et al. (2022). Engineering a lysin with Iintrinsic antibacterial activity (LysMK34) by Cecropin a fusion enhances its antibacterial properties against Acinetobacter baumannii. Appl. Environ. Microbiol. 88:e0151521. doi: 10.1128/AEM.01515-21, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

