Exploring 2D Graphene-Based Nanomaterials for Biomedical Applications: A Theoretical Modeling Perspective
- PMID: 40529861
- PMCID: PMC12168629
- DOI: 10.1002/smsc.202400505
Exploring 2D Graphene-Based Nanomaterials for Biomedical Applications: A Theoretical Modeling Perspective
Abstract
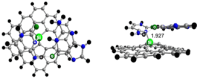
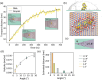
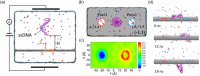
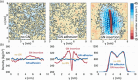
Two-dimensional (2D) graphene-based nanomaterials (GNMs) have shown potential in biomedical applications, including diagnostics, therapeutics, and drug delivery, due to their unique combination of properties such as mechanical strength, excellent electrical and thermal conductivity as well as high adsorption capacity which, combined with the ease of their surface functionalization, enable biocompatibility and bioactivity. Theoretical molecular modeling can advance our understanding of the biomedical potential of 2D graphene-based nanomaterials by providing insights into the structure, dynamics, and interactions of these nanomaterials with biological systems, at the level of detail that experiments alone cannot currently access. This perspective highlights recent computational modeling advances and challenges in examining the interactions of 2D graphene-based nanomaterials with physiologically relevant biomolecular systems, including aqueous solutions, peptides, proteins, nucleic acids, lipid membranes, and pharmaceutical drug molecules. Examples of the theoretical contributions to design of graphene-based biomaterials and devices are also provided.
Keywords: biomedicine; graphene nanomaterials; graphene oxide; molecular simulations; nanotoxicity.
© 2025 The Author(s). Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Nikzamir M., Akbarzadeh A., Panahi Y., J. Drug Delivery Sci. Technol. 2021, 61, 102316.
-
- Malode S. J., Pandiaraj S., Alodhayb A., Shetti N. P., ACS Appl. Bio Mater. 2024, 7, 752. - PubMed
LinkOut - more resources
Full Text Sources
