Regulating inflammation microenvironment and tenogenic differentiation as sequential therapy promotes tendon healing in diabetic rats
- PMID: 40529900
- PMCID: PMC12173140
- DOI: 10.1016/j.jot.2025.04.015
Regulating inflammation microenvironment and tenogenic differentiation as sequential therapy promotes tendon healing in diabetic rats
Abstract
Background: Chronic tendinopathy with diabetes mellitus (CTDM) poses significant therapeutic challenges due to persistent inflammation and impaired tenogenesis. While the supplementation of tendon stem/progenitor cells (TSPCs) has the potential to facilitate tenogenesis, premature recruitment and proliferation in inflammatory microenvironments risks fibrosis or heterotopic ossification (HO). Consequently, balancing inflammation regulation and tenogenic differentiation is critical for effective healing.
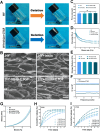
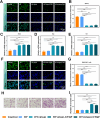
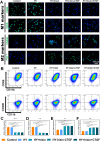
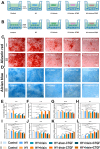
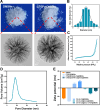
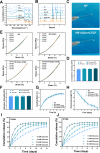
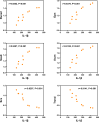
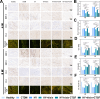
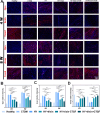
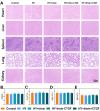
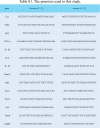
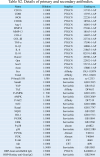
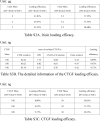
Methods: An injectable glucose-responsive dual-drug-sequential delivery hydrogel (GDSH) was developed utilizing oxidized hyaluronic acid-modified dopamine and phenylboronic acid-functionalized carboxymethyl chitosan. Dendritic mesoporous silica nanospheres (DMSNs) encapsulating irisin and connective tissue growth factor (CTGF) were incorporated into the GDSH matrix. A comprehensive characterization of the hydrogel's properties, including rheological, mechanical, adhesive, swelling/degradation, and drug release behaviors, was conducted. In vitro assessments were performed to evaluate cytocompatibility, as well as antioxidant and anti-inflammatory effects, alongside the migration, proliferation, and differentiation of TSPCs. The therapeutic efficacy was further investigated using a collagenase type I/streptozotocin-induced CTDM model in rats, with analyses conducted through histological, biomechanical, and micro-CT methods. Transcriptome sequencing and Western blot analyses were employed to elucidate the involvement of specific signaling pathways in the tissue repair process.
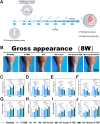
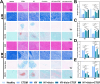
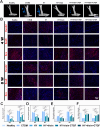
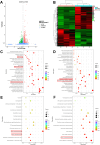
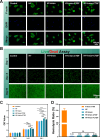
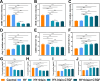
Results: The GDSH composite hydrogels possess a range of advantageous properties, including exceptional mechanical strength, optimal adhesiveness, superior biocompatibility, and appropriate swelling and degradation rates, in addition to controllable and sequential drug release capabilities. In vitro investigations revealed that these composite hydrogels exhibit antioxidant and anti-inflammatory effects, while also promoting cell proliferation and migration. Furthermore, they facilitate tenogenic differentiation and simultaneously inhibit the aberrant differentiation of TSPCs. In vivo studies demonstrated that the composite hydrogels significantly improved the morphological and biomechanical properties of injured tendons, reduced inflammation, corrected abnormal differentiation, and displayed favorable biosafety profiles. Transcriptome sequencing and Western blotting analysis indicated that the composite hydrogels repaired CTDM through the MAPK, AMPK, Smad, Hippo and PI3K/AKT signaling pathways.
Conclusion: GDSH achieves spatiotemporal control of inflammation resolution and tenogenesis via glucose-responsive sequential delivery of irisin and CTGF. This strategy restores tendon microstructure, biomechanics, and redox homeostasis in CTDM, offering a translatable platform for diabetic tendon regeneration.
The translational potential of this article: This study presents a glucose-responsive dual-drug-sequential delivery hydrogel (GDSH) designed for the treatment of chronic tendinopathy with diabetes mellitus (CTDM). This innovative approach aims to balance the regulation of inflammation and promote tenogenic differentiation. The sequential release of irisin and connective tissue growth factor (CTGF) effectively addresses the dual challenges posed by oxidative stress/inflammation and aberrant differentiation during tendon repair. The hydrogel's demonstrated biocompatibility, controlled drug release, and efficacy in restoring tendon structure and function highlight its potential for clinical translation. This platform represents a safer and more effective alternative to conventional treatments. Future research should focus on scaling up production, assessing long-term safety, and facilitating the translation of this technology into human clinical trials for the management of tendon injuries in diabetic patients.
Keywords: Chronic tendinopathy; Controllable release; Diabetes mellitus; Glucose-responsive; Inflammation microenvironment; Sequential delivery; Tenogenic differentiation.
© 2025 The Authors.
Conflict of interest statement
We would like to submit a revised original article entitled“Regulating inflammation microenvironment and tenogenic differentiation as sequential therapy promotes tendon healing in diabetic rats”, which we wish to be considered for publication in Journal of Orthopaedic Translation. No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed. In this work, we demonstrated the advantages of sequential release of irisin and CTGF in the treatment of tendon injuries, highlighting the benefits of balancing inflammation and tenogenesis.
Figures





Similar articles
-
An all-silk-based functional system promotes tendon regeneration by regulating the cell fate of TSPCs in an inflammatory microenvironment.Acta Biomater. 2025 Jun 15;200:432-451. doi: 10.1016/j.actbio.2025.05.040. Epub 2025 May 26. Acta Biomater. 2025. PMID: 40409996
-
Dumbbell-shaped hydrogel plug for annulus fibrosus repair: From material design to in vivo validation.J Orthop Translat. 2025 Jun 25;53:175-186. doi: 10.1016/j.jot.2025.06.004. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40656896 Free PMC article.
-
Quercetin attenuates tendon stem/progenitor cell senescence and promotes aged tendon repair via AKT/NF-κB/NLRP3-mediated mitophagy activation.J Adv Res. 2025 Aug 11:S2090-1232(25)00604-6. doi: 10.1016/j.jare.2025.08.013. Online ahead of print. J Adv Res. 2025. PMID: 40803494
-
Hydrogel-driven innovations for targeted delivery, immune modulation, and tissue repair in thyroid cancer therapy.Front Cell Dev Biol. 2025 Jul 25;13:1608709. doi: 10.3389/fcell.2025.1608709. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40791984 Free PMC article. Review.
-
Deciphering the therapeutic potential of Sinigrin: A promising anti-inflammatory agent for chronic disease management.Phytomedicine. 2025 Aug;144:156875. doi: 10.1016/j.phymed.2025.156875. Epub 2025 Jun 1. Phytomedicine. 2025. PMID: 40505485 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

