Targeting the NLRP3 inflammasome for calcium oxalate stones: pathophysiology and emerging pharmacological interventions
- PMID: 40529990
- PMCID: PMC12170326
- DOI: 10.3389/fphys.2025.1614438
Targeting the NLRP3 inflammasome for calcium oxalate stones: pathophysiology and emerging pharmacological interventions
Abstract
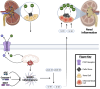
Kidney stone disease (nephrolithiasis) is a widespread condition affecting millions worldwide, with its prevalence rising due to dietary changes, obesity, and climate-related factors. The formation of kidney stones is driven by urinary solute supersaturation, metabolic abnormalities, and environmental influences. Calcium oxalate stones, the most common type, result from hypercalciuria, hyperoxaluria, and hypocitraturia, often exacerbated by high dietary protein intake and hormonal imbalances such as hyperparathyroidism. A significant complication of kidney stones is their association with chronic kidney disease (CKD). Recurrent stone formation contributes to renal scarring, urinary obstruction, and inflammation, ultimately leading to long-term kidney damage. This review explores the pivotal role of the NLRP3 inflammasome in kidney stone-related inflammation. Activated by calcium oxalate crystals and oxidative stress, NLRP3 triggers the release of pro-inflammatory cytokines (IL-1β and IL-18), exacerbating renal injury and fibrosis. Persistent NLRP3 activation is linked to CKD progression and an increased risk of end-stage renal disease. Emerging therapies targeting NLRP3 offer potential strategies to mitigate kidney stone-induced inflammation and CKD progression. Direct inhibitors such as MCC950 and CP-456773 block inflammasome activation, reducing inflammatory cytokine release. Indirect approaches, including atorvastatin and phenylbutyric acid, address oxidative stress and mitochondrial dysfunction to lower stone formation risk. While these treatments show promise in preclinical studies, further research is needed to validate their clinical efficacy. Future studies should focus on optimizing NLRP3-targeted therapies, assessing their long-term effects on kidney stone prevention and CKD progression. Combining NLRP3 inhibitors with antioxidants may enhance renal protection, providing new avenues for therapeutic intervention.
Keywords: NLRP3 inflammasome; calcium oxalate stones; chronic kidney disease; kidney stone disease; renal inflammation.
Copyright © 2025 Boldt and Di Sole.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Therapeutic potential of oxalate decarboxylase (OxDC) in the treatment of urolithiasis (kidney stone diseases).Int J Biol Macromol. 2025 Jul;318(Pt 3):145168. doi: 10.1016/j.ijbiomac.2025.145168. Epub 2025 Jun 10. Int J Biol Macromol. 2025. PMID: 40505910 Review.
-
Gut microbiota-derived indole-3-acetic acid ameliorates calcium oxalate renal stone formation via AHR/NF‑κB axis.Urolithiasis. 2025 Jul 2;53(1):134. doi: 10.1007/s00240-025-01779-0. Urolithiasis. 2025. PMID: 40601009 Free PMC article.
-
Flavonoids regulating NLRP3 inflammasome: a promising approach in alleviating diabetic peripheral neuropathy.Inflammopharmacology. 2025 May;33(5):2231-2262. doi: 10.1007/s10787-025-01729-7. Epub 2025 Apr 9. Inflammopharmacology. 2025. PMID: 40205269 Free PMC article. Review.
-
Effectiveness of Subap Plus, a Polyherbal Medicine, on 24-Hour Urinalysis and Early Morning Urine pH in Recurrent Calcium Oxalate Stone Formers: A Pilot Study.Cureus. 2025 Jul 12;17(7):e87764. doi: 10.7759/cureus.87764. eCollection 2025 Jul. Cureus. 2025. PMID: 40792340 Free PMC article.
-
Dietary advice for patients with bowel-related conditions and malabsorption.World J Urol. 2023 May;41(5):1235-1242. doi: 10.1007/s00345-023-04281-7. Epub 2023 Jan 17. World J Urol. 2023. PMID: 36648528 Review.
References
-
- Anders H. J., Suarez-Alvarez B., Grigorescu M., Foresto-Neto O., Steiger S., Desai J., et al. (2018). The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 93 (3), 656–669. 10.1016/j.kint.2017.09.022 - DOI - PubMed
-
- Boxer M. B., Shen M., Auld D. S., Wells J. A., Thomas C. J. (2010). “A small molecule inhibitor of Caspase 1,” in Probe reports from the NIH molecular libraries Program (Bethesda, MD: National Center for Biotechnology Information; ). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

