Efficacy of Janus Kinase Inhibitors in Alopecia in Jordanian Patients: A Retrospective Cohort Study
- PMID: 40530228
- PMCID: PMC12172001
- DOI: 10.7759/cureus.84321
Efficacy of Janus Kinase Inhibitors in Alopecia in Jordanian Patients: A Retrospective Cohort Study
Abstract
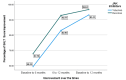
Introduction Alopecia areata (AA) is an autoimmune disorder causing non-scarring hair loss on the scalp, eyebrows, eyelashes, and other areas. Janus kinase (JAK) inhibitors have emerged as promising treatments, but data on their efficacy in Middle Eastern populations, including Jordanians, are limited. The Severity of Alopecia Tool (SALT) score is commonly used to assess disease severity, while Clinician-Reported Outcome (ClinRO) measures provide additional insights. Aim To evaluate the efficacy of JAK inhibitors in Jordanian AA patients using the SALT score as the primary outcome measure. Methods A retrospective cohort study was conducted at King Hussein Hospital, Jordanian Royal Medical Services, from January 2020 to December 2023. Medical records of AA patients aged ≥18 years treated with JAK inhibitors were reviewed. Data included demographics, disease duration, previous treatments, and adverse effects. Efficacy was assessed by the percentage change in SALT scores at six and 12 months. Statistical analyses included repeated-measures MANCOVA (Multivariate Analysis of Covariance), Chi-square, and independent t-test. A p-value <0.05 was considered significant. Results Our analysis included 57 patients, of which 31 (54.4%) received tofacitinib and 26 (45.6%) received baricitinib. A significantly higher proportion of baricitinib users had treatment durations >12 months (53.8%) compared to tofacitinib users (12.9%), while shorter durations (three to six months) were more common among tofacitinib users (41.9% vs. 15.4%; p = 0.003). Baricitinib users showed greater improvement in SALT scores between six to 12 months (92.77% vs. 82.93%; p = 0.030, partial η² = 0.084), with a trend toward greater total improvement at 12 months (96.64% vs. 93.11%; p = 0.055, partial η² = 0.067). Although not statistically significant, baricitinib showed numerically higher ClinRO improvement in eyebrows from six to 12 months (84.58% vs. 70.29%; p = 0.212) and in eyelashes (83.92% vs. 73.40%; p = 0.313), suggesting better late-stage response compared to tofacitinib. Conclusion JAK inhibitors demonstrated efficacy in Jordanian patients with alopecia areata, leading to enhanced SALT scores and noticeable hair regrowth, with baricitinib demonstrating greater improvement in SALT scores compared to tofacitinib.
Keywords: alopecia areata; baricitini; janus kinase inhibitors; salt score; tofacitinibb.
Copyright © 2025, Aldebei et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


Similar articles
-
Understanding eyebrow and eyelash involvement in patients with alopecia areata and responsiveness to treatment with baricitinib.Br J Dermatol. 2025 Jul 17;193(2):240-249. doi: 10.1093/bjd/ljaf088. Br J Dermatol. 2025. PMID: 40179237 Clinical Trial.
-
Evaluating Current and Emergent JAK Inhibitors for Alopecia Areata: A Narrative Review.Dermatol Ther (Heidelb). 2025 Aug 12. doi: 10.1007/s13555-025-01517-9. Online ahead of print. Dermatol Ther (Heidelb). 2025. PMID: 40794245 Review.
-
Efficacy and safety of different JAK inhibitors in the treatment of alopecia areata: a network meta-analysis.Front Immunol. 2023 Apr 17;14:1152513. doi: 10.3389/fimmu.2023.1152513. eCollection 2023. Front Immunol. 2023. PMID: 37138884 Free PMC article.
-
Janus Kinase Inhibitors for Alopecia Areata: A Systematic Review and Meta-Analysis.JAMA Netw Open. 2023 Jun 1;6(6):e2320351. doi: 10.1001/jamanetworkopen.2023.20351. JAMA Netw Open. 2023. PMID: 37368402 Free PMC article.
-
Characteristics and predictive values of super-responders in alopecia areata under tofacitinib treatment: a single-centre retrospective study.Clin Exp Dermatol. 2025 Jul 24;50(8):1571-1577. doi: 10.1093/ced/llaf131. Clin Exp Dermatol. 2025. PMID: 40083207
References
-
- The autoimmune basis of alopecia areata: a comprehensive review. Islam N, Leung PS, Huntley AC, Gershwin ME. Autoimmun Rev. 2015;14:81–89. - PubMed
-
- Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. Chu SY, Chen YJ, Tseng WC, et al. J Am Acad Dermatol. 2011;65:949–956. - PubMed
-
- Alopecia areata. An evaluation of 736 patients. Muller SA, Winkelmann RK. https://jamanetwork.com/journals/jamadermatology/fullarticle/528193. Arch Dermatol. 1963;88:290–297. - PubMed
LinkOut - more resources
Full Text Sources
