Therapeutic potential of adenosine receptor modulators in cancer treatment
- PMID: 40530308
- PMCID: PMC12171953
- DOI: 10.1039/d5ra02235e
Therapeutic potential of adenosine receptor modulators in cancer treatment
Abstract
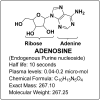
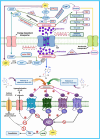
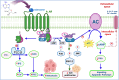
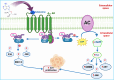
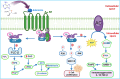
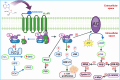
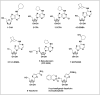
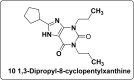
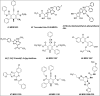
All human cells contain the universal autocoid adenosine, which interacts with four types of G protein-coupled receptors (GPCRs), namely A1, A2A, A2B, and A3 adenosine receptors (ARs). Among these receptors, A2A and A2B ARs activate adenylate cyclase, while A1 and A3 ARs suppress the adenylate cyclase activity. Adenosine-receptor interactions play a crucial role in cancer biology by modulating the immune microenvironment, which tumors exploit to create immunosuppression that promotes their growth and metastasis. When the A2A AR is activated on natural killer (NK) cells and T cells, it reduces their ability to carry out cytotoxic functions. This activation also encourages the formation of immune-suppressing cell types, such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), further weakening the immune response. Targeting adenosine receptors, particularly the A2A subtype, represents a promising therapeutic strategy. By antagonizing these receptors, it may be possible to restore T cell function, helping the body to recognize and attack cancer cells more effectively. Despite recent advancements in the discovery of novel, targeted anticancer agents, these treatments have shown limited effectiveness against metastatic tumours, complicating cancer management. Moreover, developing adenosine receptor agonists or antagonists with high target selectivity and potency remains a significant challenge, as the widespread distribution of adenosine receptors throughout the body raises concerns about off-target effects and reduced therapeutic efficacy. In order to improve outcomes for patients with advanced cancer, researchers are actively investigating safer and more efficient chemotherapy substitutes. However, drugs that activate A3 adenosine receptors and block A2A receptors are being explored as a novel approach for cancer treatment. Monoclonal antibodies and small-molecule inhibitors targeting the CD39/CD73/A2A AR axis are also being tested in clinical trials, both as standalone treatments and in combination with anti-PD-1/PD-L1 immunotherapies. This review primarily focuses on the signaling pathways and the therapeutic potential of various adenosine receptor agonists and antagonists across various cancer types, highlighting their ongoing evaluation in preclinical and clinical trials, both as monotherapies and in rational combination with immunotherapy, chemotherapy, or targeted therapies, potentially leading to the development of advanced treatments that could aid in tumor suppression.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
The Role of Immunotherapy and Immune Modulators in Hormone-Positive Breast Cancer: Implications for Localized and Metastatic Disease.J Clin Med. 2025 Jun 17;14(12):4322. doi: 10.3390/jcm14124322. J Clin Med. 2025. PMID: 40566067 Free PMC article. Review.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
-
Upcoming drug targets for kidney protective effects in chronic kidney disease.Nephrol Dial Transplant. 2025 Feb 5;40(Supplement_1):i47-i58. doi: 10.1093/ndt/gfae216. Nephrol Dial Transplant. 2025. PMID: 39907540 Free PMC article. Review.
-
PMN-MDSCs are responsible for immune suppression in anti-PD-1 treated TAP1 defective melanoma.Clin Transl Oncol. 2025 Jul;27(7):3073-3083. doi: 10.1007/s12094-024-03840-7. Epub 2025 Jan 18. Clin Transl Oncol. 2025. PMID: 39825997
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

