Induced neural stem cells ameliorate blood-brain barrier injury by modulating the calcium signaling pathway of astrocyte in cerebral ischemia-reperfusion rats
- PMID: 40530334
- PMCID: PMC12170639
- DOI: 10.3389/fcell.2025.1611226
Induced neural stem cells ameliorate blood-brain barrier injury by modulating the calcium signaling pathway of astrocyte in cerebral ischemia-reperfusion rats
Abstract
Background: Neural stem cells offer new hope for ischemic stroke patients on the basis of their potential to reverse neurological sequelae, but it is still difficult to obtain sufficient neural stem cells in the clinic. We induced human placental mesenchymal stem cells to neural stem cells (iNSCs), the therapeutic effects and possible mechanisms of iNSCs in ischemic stroke were observed in this study.
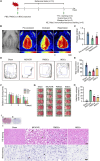
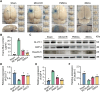
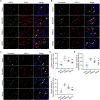
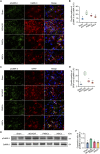
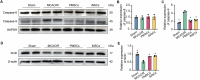
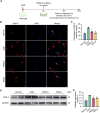
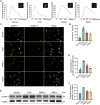
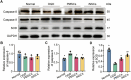
Results: Transplanted iNSCs improved neurological deficits, increased the integrity of blood-brain barrier (BBB) structure and its related proteins expression level in middle cerebral artery occlusion/reperfusion (MCAO/R) rats. The in vitro study demonstrated that iNSCs treatment inhibited Ca2+ influx in oxygen-glucose deprived (OGD)-damaged astrocytes. Additionally, iNSCs downregulated the expression level of pCaMK-II, increased the expression level of superoxide dismutase, and inhibited the expression of caspase 9 in both brain of MCAO/R rats and OGD-damaged astrocytes.
Conclusion: iNSCs transplantation improved BBB function by modulating calcium signaling pathway of astrocyte in MCAO/R rats, which proved iNSCs may be a new promising neural stem cells origin for the treatment of cerebral ischemia-reperfusion injury.
Keywords: BBB; astrocytes; calcium signaling pathways; cerebral ischemia-reperfusion; induced neural stem cells.
Copyright © 2025 Liang, Cao, Liu, Chen, Liu, Ma, Liu, Wu and Niu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
MicroRNA-29a-5p attenuates hemorrhagic transformation and improves outcomes after mechanical reperfusion for acute ischemic stroke.Noncoding RNA Res. 2025 May 28;14:96-106. doi: 10.1016/j.ncrna.2025.05.016. eCollection 2025 Oct. Noncoding RNA Res. 2025. PMID: 40529227 Free PMC article.
-
Knockdown of RUNX2 Attenuated A1 Astrocyte Overactivation, Brain Injury, and Cerebral Edema During Ischemic Stroke.Neuromolecular Med. 2025 Jun 27;27(1):48. doi: 10.1007/s12017-025-08868-8. Neuromolecular Med. 2025. PMID: 40576866
-
Hydroxysafflor yellow A attenuates the inflammatory response in cerebral ischemia-reperfusion injured mice by regulating microglia polarization per SIRT1-mediated HMGB1/NF-κB signaling pathway.Int Immunopharmacol. 2025 Feb 6;147:114040. doi: 10.1016/j.intimp.2025.114040. Epub 2025 Jan 10. Int Immunopharmacol. 2025. PMID: 39798476
-
Hair follicle stem cell-derived secretome protects astrocytes in an in vitro ischemia/reperfusion model.Anat Cell Biol. 2025 Jun 30;58(2):264-273. doi: 10.5115/acb.24.213. Epub 2025 Mar 18. Anat Cell Biol. 2025. PMID: 40097261 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Bederson J. B., Pitts L. H., Germano S. M., Nishimura M. C., Davis R. L., Bartkowski H. M. (1986). Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17 (6), 1304–1308. 10.1161/01.str.17.6.1304 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

