Consumption of the Prebiotic-Rich Chicory Taproot Contrasts the Cognitive and Motivational Consequences of Chronic Corticosterone Exposure and Modulates Gut Microbiota Composition in Mice
- PMID: 40530341
- PMCID: PMC12173009
- DOI: 10.1016/j.bpsgos.2025.100520
Consumption of the Prebiotic-Rich Chicory Taproot Contrasts the Cognitive and Motivational Consequences of Chronic Corticosterone Exposure and Modulates Gut Microbiota Composition in Mice
Abstract
Background: The human gastrointestinal tract harbors trillions of microbes that act in synergy with the brain to regulate its homeostasis and function. This interplay holds promise for innovative dietary-based interventions to support cognitive and motivational processes or contrast their decline in disease. While probiotics have traditionally been used for such interventions, several limitations have hampered their suitability and incited interest in prebiotics. Fructans represent a valid prebiotic whereby they are abundant in several vegetables (e.g., chicory taproots) and increase short-chain fatty acids (SCFAs) production via fermentation by gut microbes. SCFAs have been reported to modulate gene expression in the brain via epigenetic mechanisms. Here, we investigated whether chicory taproots may represent a strategy to contrast cognitive and motivational impairments induced by chronic corticosterone administration.
Methods: To test our hypothesis, we exposed C57BL/6 male mice (n = 18 per group) to corticosterone supplementation in drinking water and provided them with a fructan-rich diet (regular diet enriched with dried chicory taproots).
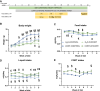
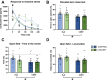
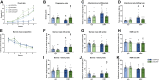
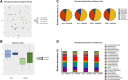
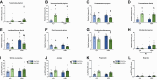
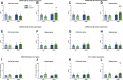
Results: Consistent with our hypothesis, chicory taproot consumption promoted the growth of selected microbial species and increased SCFA concentrations. To verify the functional role of these modulations, using a comprehensive behavioral test battery, we observed that chicory taproots contrasted the cognitive and motivational consequences of chronic corticosterone exposure. These behavioral modifications were associated with a modulation of gene expression and its epigenetic regulators in brain regions relevant for cognition and motivation.
Conclusions: These results highlight the role of prebiotics in preserving higher-order brain functions and offer insights into their therapeutic potential.
Keywords: Chronic stress; Epigenetic modulators; Executive functions; Fructans; Gut-brain axis; Short-chain fatty acids (SCFAs).
Plain language summary
The role of gut bacteria in modulating brain function is currently being studied as a potential treatment for mental illness. However, how gut bacteria regulate brain function is still unknown. Here, we explored the role of a prebiotic-rich diet (chicory taproots) in contrasting the consequences of protracted stress on emotion and cognition in mice and investigated the potential (epi)genetic mechanisms involved. Chicory consumption enhanced gut-brain communication, mitigated emotional and cognitive impairments, and increased the expression of specific genes in brain regions involved in stress and cognition. Our results may inspire innovative research that considers prebiotics in mental health therapy.
© 2025 The Authors.
Figures
References
-
- Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. - PubMed
-
- Cryan J.F., O’riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. - PubMed
LinkOut - more resources
Full Text Sources

