Targeting ApoE-KCC2 Signaling Rescues GABAergic Synaptic Dysfunction and Depression-like Behaviors in Mice
- PMID: 40530388
- PMCID: PMC12173456
- DOI: 10.34133/research.0746
Targeting ApoE-KCC2 Signaling Rescues GABAergic Synaptic Dysfunction and Depression-like Behaviors in Mice
Abstract
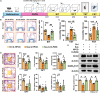
Apolipoprotein E (ApoE) has been implicated in neurodegenerative diseases; however, its function and underlying mechanisms in depression remain elusive. In this study, we employed chronic social defeat stress (CSDS) to establish a mouse model of depression and observed significantly reduced ApoE expression in the hippocampus. By leveraging ApoE knockout (ApoE-/- ) and knockdown (ApoE-KD) mouse models, we demonstrated that ApoE deficiency induced depression-like behaviors, which were closely associated with impaired GABAergic synaptic transmission and down-regulation of ApoE receptors and K+-Cl- cotransporter 2 (KCC2). In addition, we found an interaction between KCC2 and the ApoE receptor low-density lipoprotein receptor (LDLR) through coimmunoprecipitation analysis. Moreover, overexpression of ApoE or targeted activation of GABAergic neurons in the hippocampus significantly reversed depression-like behaviors in both CSDS-exposed and ApoE-KD mice. Lastly, treatment with KCC2 activators, CLP290 and CLP257, restored the expression levels of KCC2 and the GABAAR α1 subunit, significantly alleviating depression-like behaviors induced by CSDS or ApoE-KD. Together, our results elucidate the pivotal role of ApoE in the pathophysiology of depression and highlight the ApoE-KCC2 signaling pathway as a potential target for developing innovative antidepressant therapies.
Copyright © 2025 Chengyuan Xu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures










References
-
- Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, Furukawa TA, Kessler RC, Kohrt BA, Maj M, et al. Time for united action on depression: A Lancet–World Psychiatric Association Commission. Lancet. 2022;399(10328):957–1022. - PubMed
-
- Gong Q, He Y. Depression, neuroimaging and connectomics: A selective overview. Biol Psychiatry. 2015;77(3):223–235. - PubMed
-
- Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R. Novel and emerging treatments for major depression. Lancet. 2023;401(10371):141–153. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

