Immune response to and virological profile of SARS-CoV-2 before and after symptom onset in individuals vaccinated with inactivated COVID-19 vaccines
- PMID: 40530661
- PMCID: PMC12184197
- DOI: 10.1080/21645515.2025.2518654
Immune response to and virological profile of SARS-CoV-2 before and after symptom onset in individuals vaccinated with inactivated COVID-19 vaccines
Abstract
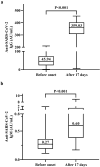
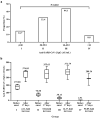
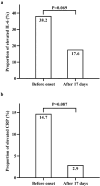
China encountered a COVID-19 pandemic shortly after lifting the zero-COVID-19 policy in December 2022. We prospectively investigated the immune responses to SARS-CoV-2 before and after symptom onset of breakthrough infection in individuals vaccinated with inactivated COVID-19 vaccines. Blood samples from 35 vaccinated participants were collected from December 16 to 20, 2022 when they had no symptoms and a second blood sample was collected from each participant from January 4 to 6, 2023. SARS-CoV-2 RNA, anti-SARS-CoV-2 IgG and IgM, interleukin-6 (IL-6), and C-reactive protein (CRP) were measured with commercially available kits respectively. During a period of average 17.0 (±1.6) days, 30 (85.7%) of 35 participants developed toxemia and respiratory symptoms and 34 (97.1%) were defined with SARS-CoV-2 infection based on positive SARS-CoV-2 RNA in pharyngeal swabs (32) and rising titers of anti-SARS-CoV-2 IgG (34). Before symptom onset, 16 (47.1%), 13 (38.2%), and 5 (14.7%) of the infected individuals already had relatively high anti-SARS-CoV-2 IgG titers, elevated IL-6 and CRP respectively. After 17.0 (±1.6) days, the median anti-SARS-CoV-2 IgG level was significantly increased (46.85 vs 359.45 AU/mL, p < .001), whereas the proportions of elevated IL-6 and CRP were each remarkably reduced. None of the participants infected with SARS-CoV-2 had detectable anti-SARS-CoV-2 IgM. In vaccinated individuals infected with SARS-CoV-2, specific anamnestic immune responses may initiate before symptom onset and robust immune response may develop in very early phase, which should be a critical mechanism to lessen the severity of COVID-19 and reduce the mortality by COVID-19 vaccination.
Keywords: COVID-19 vaccine; anamnestic immune responses; before symptom onset; brisk initiation; vaccinated individual.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Estimation of Anti-SARS-CoV-2 IgM/IgG Seroprevalence Among Non-Vaccinated and Vaccinated University Students: A Cross-Sectional Egyptian Study.Viruses. 2025 Mar 6;17(3):378. doi: 10.3390/v17030378. Viruses. 2025. PMID: 40143306 Free PMC article.
-
The effect of early COVID-19 treatment with convalescent plasma on antibody responses to SARS-CoV-2.Microbiol Spectr. 2025 Jul;13(7):e0300624. doi: 10.1128/spectrum.03006-24. Epub 2025 Jun 9. Microbiol Spectr. 2025. PMID: 40488473 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of CoronaVac vaccine in children and adolescents (Immunita-002, Brazil): A phase IV six-month follow up.Sci Rep. 2025 Jul 2;15(1):23040. doi: 10.1038/s41598-025-94596-9. Sci Rep. 2025. PMID: 40595400 Free PMC article. Clinical Trial.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
References
-
- Julian GS, Spinardi J, Díaz M, Ospina DB, Caballero N, Goularte-Silva V, Kyaw MH. Risk factors for severe COVID-19 outcomes in LATAM countries in the post-vaccination era: an analysis of national surveillance data in Argentina, Brazil, Colombia, and Mexico. J Glob Health. 2025;15:04141. doi: 10.7189/jogh.15.04141. - DOI - PMC - PubMed
-
- Zhou YH, Xu C, Tao Y, Gu M, Zhou G, Zhou W, Jin Y, Xie J, Xu B, Zhou W, et al. Incidence of SARS-CoV-2 infection in children shortly after ending zero-COVID-19 policy in China on December 7, 2022: a cross-sectional, multicenter, seroepidemiological study. Front Public Health. 2023;11:1283158. doi: 10.3389/fpubh.2023.1283158. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
