Respiratory tract antimicrobial peptides more effectively killed multiple methicillin-resistant Staphylococcus aureus and nontypeable Haemophilus influenzae isolates after disruption from biofilm residence
- PMID: 40530663
- PMCID: PMC12323582
- DOI: 10.1128/spectrum.03066-24
Respiratory tract antimicrobial peptides more effectively killed multiple methicillin-resistant Staphylococcus aureus and nontypeable Haemophilus influenzae isolates after disruption from biofilm residence
Abstract
Bacteria
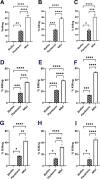
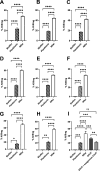
Importance: Pathogenesis of most common chronic and/or recurrent bacterial diseases (e.g., middle ear infections, urinary tract infections, rhinosinusitis, among others) can be attributed to biofilms that are canonically highly resistant to both immune effectors and antibiotics. If we treat biofilms formed by diverse human pathogens with a targeted monoclonal antibody directed at protective domains of bacterial DNA-binding proteins integral to the structural stability of the eDNA-rich biofilm matrix, they are rapidly disrupted with concomitant release of the resident bacteria. These newly released (NRel) bacteria are transiently significantly more sensitive to killing by both traditional antibiotics and human PMNs, and herein, we showed that they are also more readily killed by antimicrobial peptides. Clinically, we hope to leverage this understanding of the NRel phenotype for better medical management of these challenging infections, as well as perhaps even limit or eliminate further contribution to the global antimicrobial resistance 'pandemic'.
Keywords: DNABII; LL-37; MRSA; NRel; beta defensins; cathelicidin; nontypeable Haemophilus influenzae.
Conflict of interest statement
L.O.B. and S.D.G. are inventors of technology related to the DNABII-directed approach, rights to which have been licensed to Clarametyx Biosciences, Inc. L.O.B. and S.D.G. are Scientific Founders and Scientific Advisory Board Members, Clarametyx Biosciences, Inc. None of the other authors report any conflict of interest.
Figures
Similar articles
-
An engineered peptide derived from the innate immune effector high-mobility group box 1 disrupts and prevents dual-genera biofilms formed by common respiratory tract pathogens.FEMS Microbiol Lett. 2025 Jan 10;372:fnaf029. doi: 10.1093/femsle/fnaf029. FEMS Microbiol Lett. 2025. PMID: 40036662
-
Bacteriophage infection drives loss of β-lactam resistance in methicillin-resistant Staphylococcus aureus.Elife. 2025 Jul 10;13:RP102743. doi: 10.7554/eLife.102743. Elife. 2025. PMID: 40637714 Free PMC article.
-
Antibacterial and antibiofilm potentials of vancomycin-loaded niosomal drug delivery system against methicillin-resistant Staphylococcus aureus (MRSA) infections.BMC Biotechnol. 2024 Jul 8;24(1):47. doi: 10.1186/s12896-024-00874-1. BMC Biotechnol. 2024. PMID: 38978013 Free PMC article.
-
Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2014 Sep 9;(9):CD010010. doi: 10.1002/14651858.CD010010.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Jun 19;6:CD010010. doi: 10.1002/14651858.CD010010.pub3. PMID: 25201571 Updated.
-
Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2017 Jun 19;6(6):CD010010. doi: 10.1002/14651858.CD010010.pub3. Cochrane Database Syst Rev. 2017. PMID: 28626902 Free PMC article.
References
-
- Mokrzan EM, Ahearn CP, Buzzo JR, Novotny LA, Zhang Y, Goodman SD, Bakaletz LO. 2020. Nontypeable Haemophilus influenzae newly released (NRel) from biofilms by antibody-mediated dispersal versus antibody-mediated disruption are phenotypically distinct. Biofilm 2:100039. doi: 10.1016/j.bioflm.2020.100039 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

