Separation of telomere protection from length regulation by two different point mutations at amino acid 492 of RTEL1
- PMID: 40530700
- PMCID: PMC12203905
- DOI: 10.1093/nar/gkaf507
Separation of telomere protection from length regulation by two different point mutations at amino acid 492 of RTEL1
Abstract
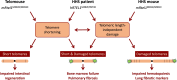
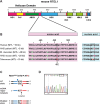
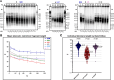
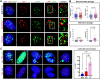
RTEL1 is an essential DNA helicase that plays multiple roles in genome stability and telomere length regulation. The ultra-long telomeres of the house mouse hinder its utility as a model for telomere-related diseases. We have previously generated a mouse model with human-length telomeres, termed "Telomouse," by substituting methionine 492 of mouse Rtel1 to a lysine (Rtel1M492K). In humans, a methionine to isoleucine mutation at this position causes the fatal telomere biology disorder Hoyeraal-Hreidarsson syndrome (HHS). Here, we introduced the Rtel1M492I point mutation into the mouse genome, generating another mouse model, which we termed "HHS mouse." The HHS mouse telomeres are not as short as those of the Telomouse but nevertheless display higher levels of telomeric DNA damage, fragility, and recombination, associated with anaphase bridges and micronuclei. The HHS mouse also exhibits aberrant hematopoiesis and pre-fibrotic alterations in the lung. These observations indicate that the two mutations at the same codon separate critical functions of RTEL1: Rtel1M492K mainly reduces the telomere length setpoint, while Rtel1M492I predominantly disrupts telomere protection. The two mouse models enable dissecting the mechanistic roles of RTEL1 and the different contributions of short telomeres and DNA damage to telomere biology disorders and genomic instability.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures






Update of
-
Separation of telomere protection from length regulation by two different point mutations at amino acid 492 of RTEL1.bioRxiv [Preprint]. 2024 Aug 25:2024.02.26.582005. doi: 10.1101/2024.02.26.582005. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Jun 6;53(11):gkaf507. doi: 10.1093/nar/gkaf507. PMID: 38464183 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 1R01DK139049-01/DK/NIDDK NIH HHS/United States
- R01 HL127349/HL/NHLBI NIH HHS/United States
- 2071/18/Israel Science Foundation
- P30 DK050306/DK/NIDDK NIH HHS/United States
- R01-HL-127349/DK/NIDDK NIH HHS/United States
- Israel-UK-Palestine GROWTH
- 2166/24/Israel Science Foundation
- R01 DK139049/DK/NIDDK NIH HHS/United States
- R01-HL-141852/DK/NIDDK NIH HHS/United States
- R01 CA249929/CA/NCI NIH HHS/United States
- 1342/23/Israel Science Foundation
- R01 HL141852/HL/NHLBI NIH HHS/United States
- R01-CA249929/DK/NIDDK NIH HHS/United States
- U01 DK134995/DK/NIDDK NIH HHS/United States
- U01-DK-134995/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

