The DEPRE'5 study: pragmatic, multicentre, five-arm, parallel-group randomised controlled trial with blinded assessment to compare treatment strategies in major depression after a failed selective serotonin reuptake inhibitor treatment
- PMID: 40530754
- PMCID: PMC12550662
- DOI: 10.1192/bjp.2025.13
The DEPRE'5 study: pragmatic, multicentre, five-arm, parallel-group randomised controlled trial with blinded assessment to compare treatment strategies in major depression after a failed selective serotonin reuptake inhibitor treatment
Abstract
Background: Selective serotonin reuptake inhibitors (SSRIs) are the first-line treatment for major depressive disorder (MDD), but initial outcomes can be modest.
Aims: To compare SSRI dose optimisation with four alternative second-line strategies in MDD patients unresponsive to an SSRI.
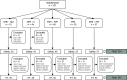
Method: Of 257 participants, 51 were randomised to SSRI dose optimisation (SSRI-Opt), 46 to lithium augmentation (SSRI+Li), 48 to nortriptyline combination (SSRI+NTP), 55 to switch to venlafaxine (VEN) and 57 to problem-solving therapy (SSRI+PST). Primary outcomes were week-6 response/remission rates, assessed by blinded evaluators using the 17-item Hamilton Depression Rating Scale (HDRS-17). Changes in HDRS-17 scores, global improvement and safety outcomes were also explored. EudraCT No. 2007-002130-11.
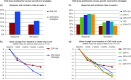
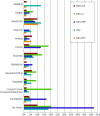
Results: Alternative second-line strategies led to higher response (28.2% v. 14.3%, odds ratio = 2.36 [95% CI 1.0-5.6], p = 0.05) and remission (16.9% v. 12.2%, odds ratio = 1.46, [95% CI 0.57-3.71], p = 0.27) rates, with greater HDRS-17 score reductions (-2.6 [95% CI -4.9 to -0.4], p = 0.021]) than SSRI-Opt. Significant/marginally significant effects were only observed in both response rates and HDRS-17 decreases for VEN (odds ratio = 2.53 [95% CI 0.94-6.80], p = 0.067; HDRS-17 difference: -2.7 [95% CI -5.5 to 0.0], p = 0.054) and for SSRI+PST (odds ratio = 2.46 [95% CI 0.92 to 6.62], p = 0.074; HDRS-17 difference: -3.1 [95% CI -5.8 to -0.3], p = 0.032). The SSRI+PST group reported the fewest adverse effects, while SSRI+NTP experienced the most (28.1% v. 75%; p < 0.01), largely mild.
Conclusions: Patients with MDD and insufficient response to SSRIs would benefit from any other second-line strategy aside from dose optimisation. With limited statistical power, switching to venlafaxine and adding psychotherapy yielded the most consistent results in the DEPRE'5 study.
Keywords: Major depressive disorder; antidepressants; psychotherapy; selective serotonin reuptake inhibitors; treatment resistant depression.
Conflict of interest statement
V.P. has received grants and served as consultant, advisor or continuing medical education (CME) speaker for AB-Biotics, Almirall, AstraZeneca, Bristol-Myers Squibb, Compass, Johnson & Johnson, Lundbeck, Medtronic, Merk, Otsuka, Pfizer, Solvay, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute) and the Servei Català de Salud (Generalitat de Catalunya). J.D.D.A has received grants from the Spanish Ministry of Health Instituto de Salud Carlos III (PI23/00469) and the Health Research and Innovation Strategic Plan (PERIS; SLT008/18/00207) of the Generalitat de Catalunya and served as an advisor or CME speaker for Johnson & Johnson, Lundbeck, Pfizer, Neuraxpharm, Esteve, Viatris and Amgen. J.B has received consulting fees from Idorsia, honoraria for speaking and teaching from GILEAD and Menarini, grants for conference registration and travel and hosting costs from Johnson & Johnson, MSD and ViiV Healthcare, with no financial or other relationship relevant to the subject of this article. A.G.P has received grants and served as a consultant, advisor or CME speaker for the following entities: Johnson & Johnson, Lundbeck, Otsuka, Angelini, Exeltis, Novartis, Rovi, Takeda, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute), the Basque Government and the European Framework Programme of Research. J.M.M. has received research funding from Johnson & Johnson, the Carlos III Health Institute and Otsuka-Lundbeck; in the last 5 years, he has had agreements or received fees as a speaker from Exeltis, Lundbeck, Angelini, Johnson & Johnson, Neuraxpharm, Pfizer, Servier, Rovi and Recordati. R.R.J has been a consultant for, spoken on activities of or received grants from Instituto de Salud Carlos III, Fondo de Investigación Sanitaria (FIS), Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid Regional Government (S2010/ BMD-2422 AGES; S2017/BMD-3740), Johnson & Johnson, Lundbeck, Otsuka, Pfizer, Ferrer, Juste, Takeda, Exeltis, Casen-Recordati, Angelini and Rovi. All other authors have no interests to declare.
Figures
References
-
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin 2003; 26: 457–94. - PubMed
-
- Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry 2007; 68: 17–25. - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am J Psychiatry 2006; 163: 1905–17. - PubMed
-
- Macqueen G, Santaguida P, Keshavarz H, Jaworska N, Levine M, Beyene J, et al. Systematic review of clinical practice guidelines for failed antidepressant treatment response in major depressive disorder, dysthymia, and subthreshold depression in adults. Can J Psychiatry 2017; 62: 11–23. - PMC - PubMed
LinkOut - more resources
Full Text Sources

