Development and characterisation of improved unifocal primary mouse lung cancer models with metastatic potential
- PMID: 40530801
- PMCID: PMC12256383
- DOI: 10.1002/path.6435
Development and characterisation of improved unifocal primary mouse lung cancer models with metastatic potential
Erratum in
-
Correction to 'Development and characterization of improved unifocal primary mouse lung cancer models with metastatic potential'.J Pathol. 2025 Nov;267(3):366. doi: 10.1002/path.6489. Epub 2025 Oct 12. J Pathol. 2025. PMID: 41077781 Free PMC article. No abstract available.
Abstract
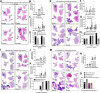
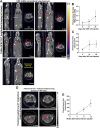
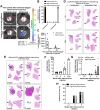
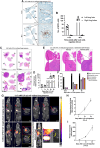
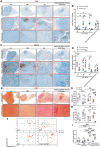
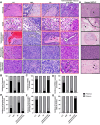
Lung cancer is the leading cause of cancer-related death globally. To better understand the biology of lung cancer, mouse models have been developed using either tail vein-injected tumour cell lines or genetically modified mice. The current gold-standard models typically present with multiple lung foci. However, although these models are widely used, their correlation with human disease are limited, as early-stage human lung cancer usually presents as a single lesion rather than multiple foci. Additionally, a major challenge of using multifocal lung tumour models is the difficulty in distinguishing primary lung tumours from intrathoracic metastasis and lethal levels of lung congestion before distant metastases develop. Here, we present a refined and detailed surgical method in which murine tumour cells [Lewis lung carcinoma (LLC), alveogenic lung carcinoma (CMT), or Kras/Trp53-KP mutant cells] were injected directly into the left lung lobe of C57BL/6 mice, or, alternatively, adenoviral-Cre or adenoviral-FlpO was administered directly into the left lung lobe of KrasLSL-G12D;Trp53fl/fl or KrasFSF-G12D;Trp53frt/frt (KP) mice, respectively. This method generated unifocal primary left lung lobe tumours with traceable spread to local and distant sites. A cross-comparison of the unifocal models described commonalties and differences between LLC, CMT, KP cells, and adenoviral-Cre or -FlpO methods in terms of timings for primary lung tumour growth and traceable spread to local and distant sites, histological analysis of CD3 and CD11b immune cell infiltration, and Picrosirius Red analysis of extracellular matrix complexity. Lastly, the frequency of clinical histopathological features typical of human lung cancer were assessed across the unifocal mouse models to provide a direct comparison with human lung cancer. Overall, this study details a refined and reproducible protocol for intralobular lung injection to generate unifocal lung cancer models that resemble key features of human lung cancer. This approach can be applied to other lung cancer initiation strategies. The cross-comparative histological analysis across the models tested here offers a valuable resource to aid researchers in selecting the most appropriate next-generation unifocal lung cancer models for their specific research needs. © 2025 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: SPECT/CT; Unifocal; histopathological features; immune infiltration; lung cancer; matrix remodelling; metastasis; non‐small cell lung cancer (NSCLC) preclinical mouse models; sodium iodide symporter.
© 2025 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Figures
References
-
- Quint LE, Tummala S, Brisson LJ, et al. Distribution of distant metastases from newly diagnosed non‐small cell lung cancer. Ann Thorac Surg 1996; 62: 246–250. - PubMed
-
- Remon J, Soria JC, Peters S, et al. Early and locally advanced non‐small‐cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol 2021; 32: 1637–1642. - PubMed
MeSH terms
Substances
Grants and funding
- C7893/A28990(A-RPandYK)/Cancer Research UK/ RadNET CRUK City of London
- Barts Charity Strategic Fund (ACK)
- MR/V009621/1(A-RP)/MRC_/Medical Research Council/United Kingdom
- WT203148/Z/16/Z(GOF)/WT_/Wellcome Trust/United Kingdom
- ALTF922-2022(SA)/European Molecular Biology Organization
- LT0010/2023-L(SA)/Human Frontier Science Program
- DRCNPG-May21|100004(BCICorefacilities)/Cancer Research UK/ RadNET CRUK City of London
- C48390/A21153(GOF)/Cancer Research UK/ RadNET CRUK City of London
- 16-1153(GOF)/AICR_/Worldwide Cancer Research/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- CTRQQR-2021\100004(JC,JF,JKandJS)/Cancer Research UK/ RadNET CRUK City of London
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

