Quorum sensing mediates morphology and motility transitions in the model archaeon Haloferax volcanii
- PMID: 40530876
- PMCID: PMC12240190
- DOI: 10.1128/mbio.00906-25
Quorum sensing mediates morphology and motility transitions in the model archaeon Haloferax volcanii
Abstract
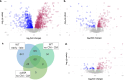
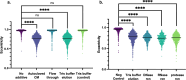
Quorum sensing (QS) is a population density-dependent mechanism of intercellular communication, whereby microbes secrete and detect signals to regulate behaviors such as virulence and biofilm formation. Although QS is well-studied in bacteria, little is known about cell-cell communication in archaea. The model archaeon Haloferax volcanii can transition from motile rod-shaped cells to non-motile disks as population density increases. In this report, we demonstrate that this transition is induced by a secreted small molecule present in cell-free conditioned medium (CM). The CM also elicits a response from a bacterial QS bioreporter, suggesting the potential for inter-domain crosstalk. To investigate the Hfx. volcanii QS response, we performed quantitative proteomics and detected significant differential abundances of 236 proteins in the presence of CM, including proteins involved in cell structure, motility, glycosylation, and two-component systems. We also demonstrate that a mutant lacking the cell shape regulatory factor DdfA does not undergo shape and motility transitions in the presence of CM, allowing us to identify protein abundance changes in the QS response pathway separate from those involved in shape and motility. In the ∆ddfA strain, only 110 proteins had significant differential abundance, and comparative analysis of these two proteomics experiments enabled us to identify proteins dependent on and independent of DdfA in the QS response pathway. Our study provides the first detailed analysis of QS pathways in any archaeon, strengthening our understanding of archaeal communication as well as providing the framework for studying intra- and interdomain crosstalk.
Importance: Understanding the complex signaling networks in microbial communities has led to many invaluable applications in medicine and industry. Yet, while archaea are ubiquitous and play key roles in nutrient cycling, little is known about the roles of archaeal intra- and interspecies cell-cell communication in environments such as the human, soil, and marine microbiomes. In this study, we established the first robust system for studying quorum sensing in archaea by using the model archaeon Haloferax volcanii. We demonstrated that different behaviors, such as cell shape and motility, are mediated by a signal molecule, and we uncovered key regulatory components of the signaling pathway. This work advances our understanding of microbial communication, shedding light on archaeal intra- and interdomain interactions, and contributes to a more complete picture of the interconnected networks of life on Earth.
Keywords: Haloferax volcanii; archaea; cell shape; intercellular signaling; motility; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Update of
-
Quorum sensing mediates morphology and motility transitions in the model archaeon Haloferax volcanii.bioRxiv [Preprint]. 2025 Jan 14:2025.01.14.633064. doi: 10.1101/2025.01.14.633064. bioRxiv. 2025. Update in: mBio. 2025 Jul 9;16(7):e0090625. doi: 10.1128/mbio.00906-25. PMID: 39868331 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

