HEY1 promotes the development and metastasis of osteosarcoma through CD44/EGFR/FAK pathway
- PMID: 40531089
- PMCID: PMC12175638
- DOI: 10.1111/jcmm.70042
HEY1 promotes the development and metastasis of osteosarcoma through CD44/EGFR/FAK pathway
Abstract
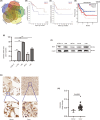
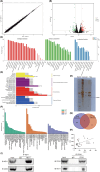
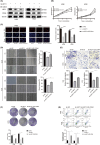
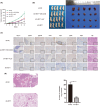
Osteosarcoma (OS) is a highly prevalent and deadly malignant tumour primarily affecting adolescents. However, the identification of new therapeutic targets remains an urgent need. The advent of bioinformatics technology has offered us a novel approach to screen key genes from diverse OS-related databases, thereby providing valuable insights into the mechanistic understanding of OS prognosis. In this study, we comprehensively integrated multiple databases to identify the crucial oncogene, HEY1, which exerts a significant impact on OS prognosis. Subsequently, we conducted a experimental validations to explore influence of HEY1 knockdown on OS cells. HEY1 exhibited significant overexpression in OS tissues and cells and its silencing resulted in a significant inhibition of proliferation. The interaction between HEY1 and CD44 was identified through transcriptome sequencing and mass spectrometry analysis. Additionally, our findings suggested that HEY1 could potentially influence the EGFR-FAK pathway. Further experiments established that HEY1 regulates the EGFR-FAK pathway via CD44, thereby influencing the biological phenotype of OS cells. These findings were subsequently validated using in vivo animal models. In summary, HEY1 demonstrated significant overexpression in both OS tissues and cells, exerting a substantial impact on the prognosis of OS.
Keywords: Bioinformatic; CD44; HEY1; focal adhesion pathway; osteosarcoma.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14(11):722‐735. - PubMed
-
- Ritter J, Bielack SS. Osteosarcoma Annals of oncology. Med Oncol. 2010;21(7):320‐325. - PubMed
-
- Meltzer PS, Helman LJ. New horizons in the treatment of osteosarcoma. N Engl J Med. 2021;385(22):2066‐2076. - PubMed
-
- Zils K, Bielack S, Wilhelm M, et al. Osteosarcoma of the mobile spine. Ann Oncol. 2013;24(8):2190‐2195. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

