Transcranial Electrical Stimulation in Treatment of Depression: A Systematic Review and Meta-Analysis
- PMID: 40531534
- PMCID: PMC12177679
- DOI: 10.1001/jamanetworkopen.2025.16459
Transcranial Electrical Stimulation in Treatment of Depression: A Systematic Review and Meta-Analysis
Abstract
Importance: The role and safety of transcranial electrical stimulation (tES) for treating depressive disorders remain under evaluation.
Objective: To evaluate tES treatment in patients with major depressive disorder (MDD) and comorbid depressive conditions.
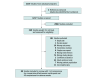
Data sources: A search of MEDLINE, Embase, Cochrane, APA PsycINFO, and Scopus databases was conducted from inception to September 17, 2024.
Study selection: Randomized clinical trials (RCTs) of adults with MDD, depression with psychiatric comorbidities (DPC), or depression with medical comorbidities (DMC), treated with transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), or transcranial random noise stimulation (tRNS), compared with sham or other treatments were included.
Data extraction and synthesis: Independent reviewers extracted data in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines, with random-effects meta-analysis used for pooling.
Main outcomes and measures: Primary outcomes were depression severity, response and remission rates, and adverse events. Standardized mean differences (SMDs) were reported for continuous outcomes, and odds ratios (ORs) were reported for categorical outcomes. Quality of evidence (QOE) was assessed using the Grading of Recommendations Assessment, Development, and Evaluation criteria.
Results: The meta-analysis included 5522 participants from 114 study groups from 88 RCTs (3198 female [58.9%]; mean [range] age, 43.1 [19.4-76.9] years). Most studies (104 study groups from 79 RCTs [91.2%]) evaluated tDCS, while 7 study groups from 6 RCTs (6.1%) evaluated tACS, and 3 study groups from 3 RCTs (2.7%) evaluated tRNS. tES was associated with reduced depressive symptoms (SMD = -0.59; 95% CI, -0.83 to -0.35; low QOE) and improvement in DMC (SMD = -1.05; 95% CI, -1.67 to -0.43; low QOE) and DPC (SMD = -0.78; 95% CI, -1.27 to -0.29; low QOE) compared with MDD (SMD = -0.22; 95% CI, -0.44 to 0.01; low QOE). tDCS was associated with reduced depression in DMC (SMD = -1.05; 95% CI, -1.70 to -0.40; very low QOE) and DPC (SMD = -0.88; 95% CI, -1.40 to -0.36; low QOE) but not MDD. tACS was associated with improved MDD symptoms (SMD = -0.58; 95% CI, -0.96 to -0.20; high QOE) and response rates (OR, 2.07; 95% CI, 1.34 to 3.19; high QOE). Combined tDCS and medication was associated with reduced symptoms (SMD = -0.51; 95% CI, -0.90 to -0.13; moderate QOE) and increased response (OR, 2.25; 95% CI, 1.08 to 4.65; high QOE) in MDD. tDCS combined with psychotherapy was not associated with improvement. Subgroup analysis showed that anodal left dorsolateral prefrontal cortex DCS was associated with improved outcomes. Mild to moderate adverse events were more frequent in tES groups.
Conclusions and relevance: In this systematic review and meta-analysis, tDCS was associated with improvement in depression among patients with DMC and DPC (with smaller benefits in MDD), tACS was associated with improved MDD outcomes (while tRNS had insufficient evidence) in smaller samples, and combined tDCS and medication was associated with improvement in depression. These findings suggest that tES is well-tolerated overall, with only mild to moderate adverse events, and that future research should optimize stimulation parameters and individualize tES interventions.
Conflict of interest statement
Figures



References
-
- Substance Use and Mental Health Services Administration. National Survey on Drug Use and Health. Published 2023. Accessed May 13, 2025. https://www.samhsa.gov/data/data-we-collect/nsduh-national-survey-drug-u...
-
- Wang J, Luo H, Schülke R, Geng X, Sahakian BJ, Wang S. Is transcranial direct current stimulation, alone or in combination with antidepressant medications or psychotherapies, effective in treating major depressive disorder? A systematic review and meta-analysis. BMC Med. 2021;19(1):319. doi: 10.1186/s12916-021-02181-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

