Long-term anti-SARS-CoV-2 antibody trajectories after neutralizing monoclonal antibody treatment
- PMID: 40531973
- PMCID: PMC12176123
- DOI: 10.1371/journal.pone.0325561
Long-term anti-SARS-CoV-2 antibody trajectories after neutralizing monoclonal antibody treatment
Abstract
Background: Neutralizing monoclonal antibodies (nMAbs) have been used to treat COVID-19 and are increasingly being used to treat other infections. However, there is concern that by neutralizing the SARS-CoV-2 virus, nMAbs may decrease the availability of antigens to the immune system, potentially impairing the endogenous polyclonal immune response and decreasing long-term immune protection.
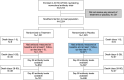
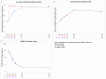
Methods: We compared 28 and 90-day anti-SARS-CoV-2 spike protein neutralization activity and anti-SARS-CoV-2 nucleocapsid response for patients hospitalized with COVID-19 infection randomized to receive nMAbs or placebo in the large platform ACTIV-3/TICO trials. We pooled results from four trials of anti-spike nMAbs. For most tested agents, measurements of the spike protein response reflect both the therapeutic and endogenous immune response. Anti-nucleocapsid levels reflect only the endogenous immune response. Data are summarized as mean differences in percent binding inhibition (anti-spike) and signal-to-cutoff (S/C) ratio (anti-nucleocapsid). Linear mixed effects models were fit to compare the longitudinal trajectory between treatment and placebo groups.
Results: Of 2,254 participants in the ACTIV-3/TICO trials modified intention-to-treat population, 2,149 (95.3%) had antibody measures at baseline and at least 1 follow-up day (day 1, 3, or 5) and were included in this analysis. Antibody measures were available for 1,556 (72.4%) participants at day 28 and 1,429 (66.5%) participants at day 90. In participants who received nMAbs, anti-spike neutralization activity was higher at day 28 (mean difference in percent binding inhibition: 7.1% [95%CI: 5.3, 8.9], p < 0.001) and day 90 (mean difference in percent binding inhibition: 7.2% [95% CI: 5.4, 9.0], p < 0.001). Anti-nucleocapsid response was similar at day 28 (mean difference in S/C ratio: 0.02 [95%CI: -0.11, 0.15], p = 0.75) and day 90 (mean difference in S/C ratio: 0.08 [95% CI: -0.05, 0.21], p = 0.22). Similar patterns were observed in all trials.
Conclusions: In patients hospitalized with COVID-19, treatment with nMAbs did not decrease long-term anti-nucleocapsid response compared to placebo, suggesting neutralizing therapies do not suppress the endogenous humoral immune response in this population.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
Author BEY has received honoraria/speaker fees from Astra-Zeneca, Gilead, Sanofi, Pfizer, Moderna (paid to their institution and outside of the submitted work). All other authors report no competing interests.
Figures
References
-
- The IMpact-RSV Study Group. Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics. 1998. Sep 1;102(3):531–7. - PubMed
-
- Food and Drug Administration. Fact Sheet for Patients, Parents, and Caregivers Emergency Use Authorization (EUA) of PEMGARDA (pemivibart) for Coronavirus Disease 2019 (COVID-19). www.fda.gov/media/177069. 2024. Accessed 2024 April 25.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

