Discovery of a functionally selective serotonin receptor (5-HT1AR) agonist for the treatment of pain
- PMID: 40531992
- PMCID: PMC12175894
- DOI: 10.1126/sciadv.adv9267
Discovery of a functionally selective serotonin receptor (5-HT1AR) agonist for the treatment of pain
Abstract
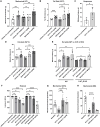
The heterotrimeric G protein-coupled serotonin receptor 5-HT1A receptor (5-HT1AR) mediates antinociception and may serve as a valuable target for the treatment of pain. Starting from a chemical library, we evolved ST171, a bitopic 5-HT1AR agonist that revealed highly potent and functionally selective Gi/o signaling without Gs activation and marginal β-arrestin recruitment. ST171 is effective in acute and chronic pain models. Cryo-electron microscopy structures of ST171 bound to 5-HT1AR in complex with the Gi protein compared to the canonical agonist befiradol bound to complexes of 5-HT1AR with Gi or Gs revealed that the ligands occupy different exo-sites. The individual binding poses are associated with ligand-specific receptor conformations that were further studied by molecular dynamics simulations, allowing us to better understand ligand bias, a phenomenon that may be crucial to the discovery of more effective and safe G protein-coupled receptor drugs.
Figures



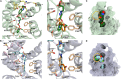


References
-
- Benyamin R., Trescot A. M., Datta S., Buenaventura R., Adlaka R., Sehgal N., Glaser S. E., Vallejo R., Opioid complications and side effects. Pain Physician 11, S105–S120 (2008). - PubMed
-
- Walsh S. L., Preston K. L., Bigelow G. E., Stitzer M. L., Acute administration of buprenorphine in humans: Partial agonist and blockade effects. J. Pharmacol. Exp. Ther. 274, 361–372 (1995). - PubMed
-
- Manglik A., Lin H., Aryal D. K., McCorvy J. D., Dengler D., Corder G., Levit A., Kling R. C., Bernat V., Hübner H., Huang X.-P., Sassano M. F., Giguère P. M., Löber S., Da D., Scherrer G., Kobilka B. K., Gmeiner P., Roth B. L., Shoichet B. K., Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190 (2016). - PMC - PubMed
-
- Nichols D. E., Nichols C. D., Serotonin receptors. Chem. Rev. 108, 1614–1641 (2008). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

