The RNA demethylase FTO promotes glutamine metabolism in clear cell renal cell carcinoma through the regulation of SLC1A5
- PMID: 40532011
- PMCID: PMC12175902
- DOI: 10.1126/sciadv.adv2417
The RNA demethylase FTO promotes glutamine metabolism in clear cell renal cell carcinoma through the regulation of SLC1A5
Abstract
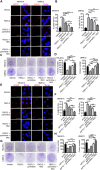
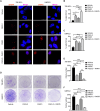
Glutamine reprogramming plays a crucial role in the growth and survival of clear cell renal cell carcinoma (ccRCC), although the mechanisms governing its regulation are still not fully understood. We demonstrate that the RNA demethylase fat mass and obesity-associated gene (FTO) drives glutamine reprogramming to support ccRCC growth and survival. Genetic and pharmacologic inhibition of FTO in ccRCC cells impaired glutamine-derived reductive carboxylation, depleted pyrimidines, and increased reactive oxygen species. This led to increased DNA damage and reduced survival, which could be rescued by pyrimidine nucleobases or the antioxidant N-acetylcysteine. Mechanistically, FTO demethylates the glutamine transporter solute carrier family 1 member 5 (SLC1A5) messenger RNA to promote its expression. Restoration of SLC1A5 expression in FTO-knockdown cells rescued metabolic and survival defects. FTO inhibition reduced ccRCC tumor xenograft and PDX growth under the renal capsule. Our findings indicate that FTO is an epitranscriptomic regulator of ccRCC glutamine reprogramming and highlight the therapeutic potential of targeting FTO for the treatment of ccRCC.
Figures

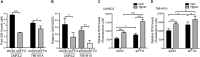


References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018). - PubMed
-
- Hutson T. E., Thoreson G. R., Figlin R. A., Rini B. I., The evolution of systemic therapy in metastatic renal cell carcinoma. Am. Soc. Clin. Oncol. Educ. Book 35, 113–117 (2016). - PubMed
-
- Rini B. I., Campbell S. C., Escudier B., Renal cell carcinoma. Lancet 373, 1119–1132 (2009). - PubMed
-
- Siegel R. L., Miller K. D., Fuchs H. E., Jemal A., Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022). - PubMed

