Microglia-specific NF-κB signaling is a critical regulator of prion-induced glial inflammation and neuronal loss
- PMID: 40532025
- PMCID: PMC12185024
- DOI: 10.1371/journal.ppat.1012582
Microglia-specific NF-κB signaling is a critical regulator of prion-induced glial inflammation and neuronal loss
Abstract
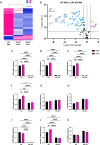
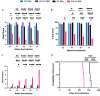
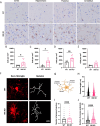
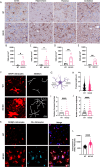
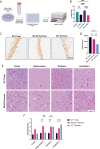
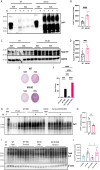
Prion diseases are a group of rare and fatal neurodegenerative diseases caused by the cellular prion protein, PrPC, misfolding into the infectious form, PrPSc, which forms aggregates in the brain. This leads to activation of glial cells, neuroinflammation, and irreversible neuronal loss, however, the role of glial cells in prion disease pathogenesis and neurotoxicity is poorly understood. Microglia can phagocytose PrPSc, leading to the release of inflammatory signaling molecules, which subsequently induce astrocyte reactivity. Animal models show highly upregulated inflammatory molecules that are a product of the Nuclear Factor-kappa B (NF-κB) signaling pathway, suggesting that this is a key regulator of inflammation in the prion-infected brain. The activation of the IκB kinase complex (IKK) by cellular stress signals is critical for NF-κB-induced transcription of a variety of genes, including pro-inflammatory cytokines and chemokines, and regulators of protein homeostasis and cell survival. However, the contribution of microglial IKK and NF-κB signaling in the prion-infected brain has not been evaluated. Here, we characterize a primary mixed glial cell model containing wild-type (WT) astrocytes and IKK knock-out (KO) microglia. These cultures show a near ablation of microglia compared to WT mixed glial cultures, highlighting the role of IKK in microglial survival and proliferation. We show that, when exposed to prion-infected brain homogenates, NF-κB-associated genes are significantly downregulated, but prion accumulation is significantly increased, in mixed glial cultures containing minimal microglia. Mice with IKK KO microglia show rapid disease progression when intracranially infected with prions, characterized by an increased density of activated microglia and reactive astrocytes, development of spongiosis, and accelerated loss of hippocampal neurons and associated behavioral deficits. These animals display clinical signs of prion disease early and have a 22% shorter life expectancy compared to infected wild-type mice. Intriguingly, PrPSc accumulation was significantly lower in the brains of terminal animals with IKK KO microglia compared to terminal WT mice, suggesting that accelerated disease is independent of PrPSc accumulation, highlighting a glial-specific pathology. Together, these findings present a critical role for microglial IKK and NF-κB signaling in host protection against prion disease.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Downregulation of STAT3 transcription factor reverses synaptotoxic phenotype of reactive astrocytes associated with prion diseases.Acta Neuropathol Commun. 2025 May 15;13(1):101. doi: 10.1186/s40478-025-02028-6. Acta Neuropathol Commun. 2025. PMID: 40375298 Free PMC article.
-
Spreading depolarization triggers pro- and anti-inflammatory signalling: a potential link to headache.Brain. 2025 Jul 7;148(7):2522-2536. doi: 10.1093/brain/awaf015. Brain. 2025. PMID: 39823578
-
Orexin-A Attenuates the Inflammatory Response in Sepsis-Associated Encephalopathy by Modulating Oxidative Stress and Inhibiting the ERK/NF-κB Signaling Pathway in Microglia and Astrocytes.CNS Neurosci Ther. 2024 Nov;30(11):e70096. doi: 10.1111/cns.70096. CNS Neurosci Ther. 2024. PMID: 39508266 Free PMC article.
-
Neurotrophomodulatory effect of TNF-α through NF-κB in rat cortical astrocytes.Cytotechnology. 2025 Feb;77(1):37. doi: 10.1007/s10616-024-00698-z. Epub 2025 Jan 5. Cytotechnology. 2025. PMID: 39776978 Review.
-
Single-incision sling operations for urinary incontinence in women.Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD008709. doi: 10.1002/14651858.CD008709.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2023 Oct 27;10:CD008709. doi: 10.1002/14651858.CD008709.pub4. PMID: 28746980 Free PMC article. Updated.
References
-
- Rocha SM, Kirkley KS, Chatterjee D, Aboellail TA, Smeyne RJ, Tjalkens RB. Microglia-specific knock-out of NF-kappaB/IKK2 increases the accumulation of misfolded alpha-synuclein through the inhibition of p62/sequestosome-1-dependent autophagy in the rotenone model of Parkinson’s disease. Glia. 2023. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

