Adherence to Physical Activity and Incident Mobility Disability in Older Adults With Mobility Limitations
- PMID: 40532064
- PMCID: PMC12176070
- DOI: 10.1002/jcsm.13870
Adherence to Physical Activity and Incident Mobility Disability in Older Adults With Mobility Limitations
Abstract
Background: Preservation of mobility independence is a primary goal in older adults with physical frailty and sarcopenia (PF&S). Interventions based on the combination of physical activity (PA) and nutritional counselling have been indicated as strategies for the management of this condition, although their effectiveness is not confirmed in all investigations. A possible explanation for this uncertain scenario relies in the impact of the adherence to PA interventions. Hence, the present study investigated the impact of the adherence to PA sessions on the incidence of mobility disability in older adults with PF&S.
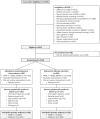
Methods: This is a secondary analysis of an evaluator blinded, randomised controlled trial, developed in 16 clinical sites across 11 European countries, from January 2016 to 31 October 2019. Participants were community-dwelling older adults (70+ years) with PF&S enrolled in the SPRINTT trial (NCT02582138). PF&S was operationalised as having a total score from 3 to 9 on the short physical performance battery (SPPB), low appendicular lean mass and ability to complete the 400-m walk test in < 15 min. Data from participants allocated to a multicomponent intervention (PA with technological support plus nutritional counselling) and a healthy ageing lifestyle education programme (control group) were analysed. Adherence to PA was assessed based on the number of weekly sessions attended. According to recommendations of the American College of Sports Medicine, adherence was categorised as below recommendations (< 2 sessions/week, BR), meeting recommendations (2-3 sessions/week, MR), and above recommendations (> 3 sessions/week, AR). The primary outcome was incident mobility disability, operationalised as incident inability to complete the 400-m walk test in < 15 min during up to 36 months of follow-up.
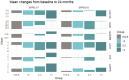
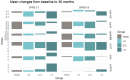
Results: Data of 1444 participants (mean age 79.3 years, 72.6% women) were analysed. In those with SPPB scores of 3-7, MR and AR groups had lower risk of mobility disability compared with controls [MR HR (95% CI): 0.57 (0.41-0.78), p = 0.001; AR HR (95% CI): 0.33 (0.23-0.46), p < 0.001] and BR groups [MR: HR (95% CI): 0.48 (0.34-0.69), p < 0.001; AR: HR (95% CI): 0.27 (0.18-0.38), p < 0.001] in a dose-dependent manner. In those with SPPB scores of 8 or 9, the BR group had a higher risk of mobility disability than controls. MR and AR groups had a lower risk of mobility disability than the BR group.
Conclusions: In older adults with PF&S, adherence to PA recommendations is associated with lower incidence of mobility disability. This benefit depends on the degree of adherence as well as baseline physical performance.
Trial registration: ClinicalTrials.gov NCT02582138.
Keywords: disability; frailty; frequency; physical activity; sarcopenia.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Ferrari A. J., Santomauro D. F., Aali A., et al., “Global Incidence, Prevalence, Years Lived With Disability (YLDs), Disability‐Adjusted Life‐Years (DALYs), and Healthy Life Expectancy (HALE) for 371 Diseases and Injuries in 204 Countries and Territories and 811 Subnational Locations, 1990‐2021: A Systematic Analysis for the Global Burden of Disease Study 2021,” Lancet 403 (2024): 2133–2161. - PMC - PubMed
-
- The Lancet Healthy Longevity , “The Decade of Healthy Ageing: Progress and Challenges Ahead,” Lancet Healthy Longevity 5 (2024): e1. - PubMed
-
- LIFE Study Investigators , Pahor M., Blair S. N., et al., “Effects of a Physical Activity Intervention on Measures of Physical Performance: Results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE‐P) Study,” Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 61 (2006): 1157–1165. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

