Development of CPA-Catalyzed β-Selective Reductive Amination of Cardenolides for the Synthesis and Biological Evaluation of Hydrolytically Stable Analogs
- PMID: 40532177
- PMCID: PMC12224169
- DOI: 10.1002/chem.202501552
Development of CPA-Catalyzed β-Selective Reductive Amination of Cardenolides for the Synthesis and Biological Evaluation of Hydrolytically Stable Analogs
Abstract
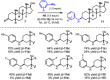
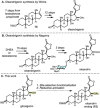
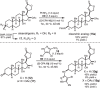
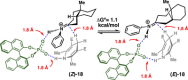
This article describes the development of novel, hydrolytically stable cardiotonic steroid analogs featuring a 3β-amine moiety instead of the commonly found 3β-carbohydrates such as oleandrose. To establish the desired 3β-configuration stereoselectively, a new method based on chiral phosphoric acid-controlled diastereoselective reductive amination with Hantzsch esters was developed. This method utilizes readily available unsubstituted (S)-BINOL-based hydrogen phosphate as the catalyst, enabling the synthesis of 13 different 5β-androsterone and digitoxigenin analogs with up to 36:1 β:α diastereoselectivity. Additionally, this strategy was applied to generate two novel oleandrigenin analogs 15a and 15g in 3 steps from readily available gitoxigenin. The synthetic analogs were subjected to the NCI-60 human tumor cell lines screen, and several different digitoxigenin derivatives with tumor cell growth inhibitory power in submicromolar range were identified. The subsequent in vitro evaluation of digitoxigenin and oleandrin derivatives 13a, 13g, 15a, and 15g demonstrated that these four analogs reduced steady-state ATP1A1 levels in T98G cells in the 12-96 nM range. Interestingly, only the oleandrin analog 15g lowered also steady-state levels of the cellular prion protein (PrPC), the main therapeutic target for the treatment of prion diseases.
Keywords: analog; anticancer activity; cardiotonic steroid; chiral phosphoric acid; diastereoselective; reductive amination.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Injected corticosteroids for treating plantar heel pain in adults.Cochrane Database Syst Rev. 2017 Jun 11;6(6):CD009348. doi: 10.1002/14651858.CD009348.pub2. Cochrane Database Syst Rev. 2017. PMID: 28602048 Free PMC article.
-
Selective Oxidation of Disparate Functional Groups Mediated by a Common Aspartic Acid-Based Peptide Catalyst Platform.Acc Chem Res. 2025 Jul 1;58(13):2072-2087. doi: 10.1021/acs.accounts.5c00247. Epub 2025 Jun 18. Acc Chem Res. 2025. PMID: 40530828
References
-
- Withering W., An Account of the Foxglove and Some of ist Medical Uses, G.G.J. and Robinson, Birmingham: 1785.
-
- a) Mijatovic T., Van Quaquebeke E., Delest B., Debeir O., Darro F., Kiss R., Biochim. Biophys. Acta (BBA) – Rev. Cancer, 2007, 1776 32; - PubMed
- b) Babula P., Masarik M., Adam V., Provaznik I., Kizek R., Anti‐Cancer Agents Med. Chem. 2013, 13, 1069; - PubMed
- c) Zanchett Scheider N. F., Cerella C., Oliveira Simões C. M., Diederich M., Molecules 2017, 22, 1932.
-
- a) Mehrabian M., Wang X., Eid S., Yan B. Q., Grinberg M., Siegner M., Sackmann C., Sulman M., Zhao W., Williams D., Schmitt‐Ulms G., PLoS One 2022, 17, e0270915; - PMC - PubMed
- b) Eid S., Zerbes T., Williams D., Wang X., Sackmann C., Meier S., Dulin O. N. P. N., Schmitt‐Ulms G., Int. J. of Mol. Sci. 2022, 23, 14823; - PMC - PubMed
- c) Eid S., Zhao W., Williams D., Nasser Z., Griffin J., Nagorny P., Schmitt‐Ulms G., PLoS One 2024, 19, e0308821; - PMC - PubMed
- d) Markina A. A., Kazanskaya R. B., Timoshina J. A., Zavialov V. A., Abaimov D. A., Volnova A. B., Fedorova T. N., Gainetdinov R. R., Lopachev A. V., Biomedicines 2023, 11, 1820. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 852085/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- R35 GM136341/GM/NIGMS NIH HHS/United States
- CIHR #202209PJT/Canadian Institutes for Health Research
- CIHR#202209PJT/Creutzfeldt-Jakob Disease Foundation
- 852078/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- 852081/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- 852077/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- 852082/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- GM136341/GM/NIGMS NIH HHS/United States
- 852083/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- 852079/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- 852084/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- 852080/National Cancer Institute Development Therapeutics Program (NCI/DTP)
- CHE-1955069/Directorate for Mathematical and Physical Sciences
- GM136341/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

