Unraveling Bifurcating Pathways for CO and HCOOH Formation: Insights from Stopped-Flow FTIR Spectroscopy of a Second-Sphere Modified Mn Catalyst
- PMID: 40532193
- PMCID: PMC12232292
- DOI: 10.1021/jacs.5c04274
Unraveling Bifurcating Pathways for CO and HCOOH Formation: Insights from Stopped-Flow FTIR Spectroscopy of a Second-Sphere Modified Mn Catalyst
Abstract
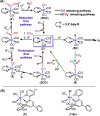
Manganese bipyridine tricarbonyl complexes show high efficiency and selectivity in electrochemical CO2 reduction (e-CO2RR) to CO. Efforts to shift selectivity toward HCOOH have been made by introducing second-sphere hydroxyl or amine functional groups and using amines or proton-coupled electron transfer (PCET) mediators. However, the direct spectroscopic evidence for the bifurcation pathways leading to CO and HCOOH remained elusive. Using stopped-flow mixing with decamethyl cobaltocene reductant and time-resolved infrared (TRIR) spectroscopy, we identified, for the first time, the key intermediates in this bifurcation pathway for an Mn complex with second-sphere hydroxyl groups in real time under catalytic conditions. The measured rate constants align with reported TOF values from electrochemical studies, validating the relevance of the results to e-CO2RR conditions. Our findings reveal that HCOOH production involves proton transfer from hydroxyl groups to the doubly reduced Mn center, forming the Mn-hydride intermediate, followed by CO2 insertion, leading to the Mn-formate intermediate. However, the inability of the resulting phenolate to rebind protons from weak acids like water leads to rapid catalyst degradation, limiting sustained catalysis. This work provides mechanistic insights and paves the way for designing molecular catalysts with enhanced selectivity and stability for HCOOH production during e-CO2RR.
Figures






Similar articles
-
A stability strategy for doped modified bismuth sulfide in CO2RR for reducing CO2 to HCOOH.J Colloid Interface Sci. 2025 Nov 15;698:138013. doi: 10.1016/j.jcis.2025.138013. Epub 2025 May 27. J Colloid Interface Sci. 2025. PMID: 40466598
-
Organic Synthesis Away from Equilibrium: Contrathermodynamic Transformations Enabled by Excited-State Electron Transfer.Acc Chem Res. 2024 Jul 2;57(13):1827-1838. doi: 10.1021/acs.accounts.4c00227. Epub 2024 Jun 21. Acc Chem Res. 2024. PMID: 38905487 Free PMC article.
-
Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children.Cochrane Database Syst Rev. 2012 Jan 18;1:CD008965. doi: 10.1002/14651858.CD008965.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Apr 10;(4):CD008965. doi: 10.1002/14651858.CD008965.pub4. PMID: 22258996 Updated.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
References
-
- Wagner A., Sahm C. D., Reisner E.. Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction. Nat. Catal. 2020;3(10):775–786. doi: 10.1038/s41929-020-00512-x. - DOI
LinkOut - more resources
Full Text Sources

