Transcranial alternating current stimulation for treating spinocerebellar ataxia type 3: A randomized controlled trial
- PMID: 40532663
- PMCID: PMC12208327
- DOI: 10.1016/j.xcrm.2025.102162
Transcranial alternating current stimulation for treating spinocerebellar ataxia type 3: A randomized controlled trial
Abstract
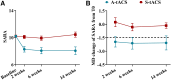
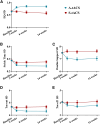
There are no specific treatments for spinocerebellar ataxia type 3 (SCA3), a neurodegenerative disease causing cerebellar dysfunction. Transcranial alternating current stimulation (tACS) can improve cerebellar motor functions, and it has been shown to be safe and effective in treating neurological diseases. This randomized controlled trial (RCT) explored the effects of tACS on SCA3 patients. Participants received either 40-min, 70 Hz, 2 mA tACS or sham stimulation daily for 2 weeks. The primary outcome was met by 80% of the active-tACS group (32/40) and 10% of the sham group (4/40). The active group also showed significantly greater reductions in the Scale for Assessment and Rating of Ataxia (SARA) scores. No serious adverse events occurred, indicating high safety. Therefore, tACS is effective, safe, and feasible for treating SCA3. The study is registered at ClinicalTrials.gov (NCT05557786).
Keywords: functional connectivity; non-invasive brain stimulation; randomized sham-controlled trial; spinocerebellar ataxia type 3; transcranial alternating current stimulation.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Pedroso J.L., França M.C., Jr., Braga-Neto P., D'Abreu A., Saraiva-Pereira M.L., Saute J.A., Teive H.A., Caramelli P., Jardim L.B., Lopes-Cendes I., Barsottini O.G.P. Nonmotor and extracerebellar features in Machado-Joseph disease: a review. Mov. Disord. 2013;28:1200–1208. doi: 10.1002/mds.25513. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

