Changes in cardiovascular disease risk, lung function and other clinical health outcomes when people who smoke use e-cigarettes to reduce cigarette smoking: an exploratory analysis from a randomised placebo-controlled trial
- PMID: 40533223
- PMCID: PMC12182122
- DOI: 10.1136/bmjopen-2024-098005
Changes in cardiovascular disease risk, lung function and other clinical health outcomes when people who smoke use e-cigarettes to reduce cigarette smoking: an exploratory analysis from a randomised placebo-controlled trial
Abstract
Objectives: To examine changes in cardiovascular disease (CVD) risk factors, lung function and clinical laboratory markers among people who smoke who used e-cigarettes to reduce their cigarette smoking.
Design: Four-arm, parallel-group, double-blind, randomised placebo-controlled trial.
Setting: Two sites-Virginia Commonwealth University (Richmond, Virginia, USA) and Penn State University, College of Medicine (Hershey, Pennsylvania, USA).
Participants: Adults (n=520) aged 21-65 years who smoked at least 10 cigarettes per day, had an expired-air carbon monoxide reading of >9 parts per million at baseline and were interested in reducing their cigarette consumption.
Interventions: E-cigarettes with 0, 8 or 36 mg/mL nicotine liquid concentration or a cigarette substitute.
Primary outcome measures: CVD risk factors (blood lipids, C-reactive protein, blood pressure, heart rate, waist-to-hip ratio, body mass index and INTERHEART risk score), lung function (spirometry indices, and pulmonary symptoms and functional state using the Clinical Chronic Obstructive Pulmonary Disorder Questionnaire), and other clinical laboratory markers (complete blood count and complete metabolic panel).
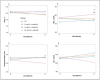
Results: At 6 months, the use of nicotine e-cigarettes caused no significant between-group differences for most measures. However, participants randomised to the 36 mg/mL e-cigarette condition had significantly higher levels of high-density lipoprotein (HDL) (p=0.003 unadjusted, p=0.002 adjusted) and lower levels of low-density lipoprotein (LDL) (p=0.044 adjusted) and cholesterol/HDL ratio (p=0.034 unadjusted, p=0.026 adjusted) compared with the cigarette substitute condition. Also, those in the 36 mg/mL e-cigarette condition had higher HDL levels than those in the 0 mg/mL condition (p=0.016 unadjusted, p=0.019 adjusted).
Conclusions: Participants randomised to the highest nicotine e-cigarette condition showed modest improvements in some measures of blood lipids (eg, increased HDL, and reduced LDL and cholesterol/HDL ratio) as compared with a non-aerosol cigarette substitute among individuals attempting to reduce their cigarette smoking. Future studies of e-cigarettes for smoking cessation would benefit from including these measures to further explore the results found in this study.
Trial registration number: NCT02342795.
Keywords: PUBLIC HEALTH; Randomized Controlled Trial; Tobacco Use.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J and Cypress Bioscience. LK currently serves as a member of the editorial board for BMJ Open but was not involved in the editorial review or decision-making process for this study.
Figures
References
-
- Adjaye-Gbewonyo D. National health interview survey early release program. 2021
-
- Norris T. Early release of selected estimates based on data from the 2023 national health interview survey. 2023
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
