Patient-informed outpatient intervention to improve pregnancy outcomes through connections to social services: protocol for the BETTER randomised controlled trial
- PMID: 40533225
- PMCID: PMC12182209
- DOI: 10.1136/bmjopen-2024-096119
Patient-informed outpatient intervention to improve pregnancy outcomes through connections to social services: protocol for the BETTER randomised controlled trial
Abstract
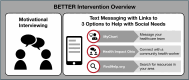
Introduction: A substantial portion of the 3.6 million births per year in the USA (approximately 25%-30%) occur in the context of adverse pregnancy outcomes (APOs), including preterm birth (PTB), hypertensive disorders of pregnancy (HDP) and small-for-gestational-age (SGA) birth. Black individuals have a 2-3-fold higher risk of APOs and a similarly increased risk of maternal morbidity and mortality compared with White individuals. Adverse social determinants of health (SDoH) are at the root of this disparity and contribute to it through multiple mechanisms. Maternal anaemia is an upstream factor associated with severe maternal morbidity, maternal mortality and other APOs and is also associated with adverse SDoH. Effectively and efficiently addressing social needs arising from adverse SDoH in the obstetric setting can be difficult due to varying patient preferences, resource accessibility and clinic workflows. The Better Birth Outcomes Through Technology, Education and Reporting (BETTER) intervention attempts to account for these barriers by encouraging patients to address social needs through motivational interviewing and by sending recurring text messages that provide links to multiple kinds of social service resources.
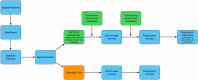
Methods and analysis: We will use a two-arm randomised controlled trial to evaluate the effects of providing patients with a motivational interviewing session and text messages with links to multiple resources to address their social needs compared with patients receiving usual care. We will recruit 550 pregnant individuals less than 21 weeks of gestation from two university prenatal clinics that primarily serve Medicaid-covered individuals in an urban city in the Midwestern USA. We will assess whether the intervention reduces the primary outcome of maternal anaemia measured as haemoglobin <11.0 g/dL at delivery as well as important secondary outcomes, including PTB, HDP and SGA. We will also explore patient-reported outcomes, including how the intervention impacts patient global health, activation, self-efficacy, perceived stress, anxiety, depression and communication with their providers. A subgroup of participants from the intervention group will be interviewed up to 12 weeks postpartum about their experiences.
Ethics and dissemination: The Ohio State University (OSU) Institutional Review Board (IRB) approved this study (IRB: 2023H0065; date: 21 February 2024). All protocol amendments will be communicated for approval to the OSU IRB. We plan to share results in peer-reviewed journal articles and academic conferences as well as with the larger American Heart Association (AHA) Pregnancy, Postpartum and Postnatal Health: Enhancing Quality and Access to Achieve Equitable Maternal and Infant Health (P3 EQUATE) Network on maternal health, including clinicians, community members and social service providers. Following the trial, we will make deidentified data publicly available in compliance with AHA policy and federal regulations.
Trial registration number: NCT06261398.
Keywords: Health Equity; OBSTETRICS; Postpartum Period; Pragmatic Clinical Trial; Pregnancy; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Mobile phone messaging for preventive health care.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007457. doi: 10.1002/14651858.CD007457.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235643 Free PMC article.
-
Effectiveness of Kushal Maa, a group-based mhealth interactive education and social support intervention for maternal and neonatal health outcomes: study protocol for a multisite randomised controlled trial in India.BMJ Open. 2025 Jun 27;15(6):e104213. doi: 10.1136/bmjopen-2025-104213. BMJ Open. 2025. PMID: 40578858 Free PMC article.
-
Integration of a Patient-Centered mHealth Intervention (Support-Moms) Into Routine Antenatal Care to Improve Maternal Health Among Pregnant Women in Southwestern Uganda: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2025 Mar 19;14:e67049. doi: 10.2196/67049. JMIR Res Protoc. 2025. PMID: 40105879 Free PMC article.
-
Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes.Cochrane Database Syst Rev. 2018 Jul 24;7(7):CD010564. doi: 10.1002/14651858.CD010564.pub2. Cochrane Database Syst Rev. 2018. PMID: 30039871 Free PMC article.
-
Mobile phone messaging for facilitating self-management of long-term illnesses.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD007459. doi: 10.1002/14651858.CD007459.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235644 Free PMC article.
References
-
- National vital statistics reports volume 72. 2023
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
