Quantitative Measurement of Tau Burden in a Dual-Time-Window Dynamic PET Imaging Protocol with [18F]MK6240
- PMID: 40533354
- PMCID: PMC12320581
- DOI: 10.2967/jnumed.125.270165
Quantitative Measurement of Tau Burden in a Dual-Time-Window Dynamic PET Imaging Protocol with [18F]MK6240
Abstract
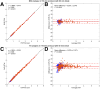
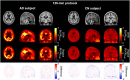
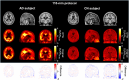
This study aimed to test and validate a dual-time-window (DTW) protocol for 6-(fluoro-18F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([18F]MK6240) dynamic PET imaging in experimental datasets acquired in human subjects. Methods: DTW protocols were tested and validated in datasets previously collected in 25 participants: 13 were cognitively normal, 10 had mild cognitive impairment, and 2 had Alzheimer disease. Participants underwent full 120-min [18F]MK6240 dynamic PET scans as well as structural MRI. Intermediary 3-dimensional volumes were removed from the acquired dynamic PET images to emulate DTW acquisitions consisting of an early phase and a late phase. Five break durations (30, 40, 50, 60, and 70 min) were investigated to determine the optimal break for 2 study durations (120 and 110 min). Regional brain time-activity curves were extracted using atlases available in the Montreal Neurologic Institute template space and using the FreeSurfer parcellation. Interpolation strategies were tested to recover the missing time points. Distribution volume ratio (DVR) estimates obtained from the DTW time-activity curves were compared with those obtained from the full time-activity curves as reference. Parametric maps were generated for the selected protocol and evaluated. Results: The correlation and agreement between DVR values obtained from the DTW method and the full time-activity curves were overall very good. The DTW protocol with a 60-min break using a biexponential model fit as the interpolation method provided the best compromise between practicality and quantitative accuracy. The mean differences between this DTW and the full acquisition, averaged across brain regions and all subjects, were less than 1% with a corresponding SD of less than 4%, and DVR estimates were not statistically different from those obtained from the full acquisition (P > 0.05). DVR parametric images were visually and quantitatively consistent with those obtained from the full acquisition. Conclusion: This study presents strong support for the use of a DTW protocol with [18F]MK6240. Such a protocol would be well suited to allow for both quantification of tau and derivation of an index of cerebral perfusion while reducing patient discomfort and increasing scanning efficiency in comparison to a full dynamic acquisition.
Keywords: Alzheimer disease; PET; [18F]MK6240; dual-time-window imaging; tau imaging.
© 2025 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


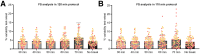


Similar articles
-
Measurement of Cerebral Perfusion Indices from the Early Phase of [18F]MK6240 Dynamic Tau PET Imaging.J Nucl Med. 2023 Jun;64(6):968-975. doi: 10.2967/jnumed.122.265072. Epub 2023 Mar 30. J Nucl Med. 2023. PMID: 36997330 Free PMC article.
-
[18F]MK-6240 Radioligand Delivery Indices as Surrogates of Cerebral Perfusion: Bias and Correlation Against [15O]Water.J Nucl Med. 2025 Mar 3;66(3):410-417. doi: 10.2967/jnumed.124.268701. J Nucl Med. 2025. PMID: 39947916 Free PMC article.
-
¹⁸F-FDG PET for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2015 Jan 28;1(1):CD010632. doi: 10.1002/14651858.CD010632.pub2. Cochrane Database Syst Rev. 2015. PMID: 25629415 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
18F PET with florbetapir for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Nov 22;11(11):CD012216. doi: 10.1002/14651858.CD012216.pub2. Cochrane Database Syst Rev. 2017. PMID: 29164603 Free PMC article.
References
-
- Walji AM, Hostetler ED, Selnick H, et al. Discovery of 6-(fluoro-18 F)-3-(1 H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([18F]-MK-6240): a positron emission tomography (PET) imaging agent for quantification of neurofibrillary tangles (NFTs). J Med Chem. 2016;59:4778–4789. - PubMed
-
- Hostetler ED, Walji AM, Zeng Z, et al. Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human neurofibrillary tangles. J Nucl Med. 2016;57:1599–1606. - PubMed
-
- Lohith TG, Bennacef I, Vandenberghe R, et al. Brain imaging of Alzheimer dementia patients and elderly controls with 18F-MK-6240, a PET tracer targeting neurofibrillary tangles. J Nucl Med. 2019;60:107–114. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
