Synthetic Study of Kosinostatin Aglycon: Synthesis of the ABCDEFG Ring Skeleton
- PMID: 40533411
- PMCID: PMC12235639
- DOI: 10.1021/acs.joc.5c00886
Synthetic Study of Kosinostatin Aglycon: Synthesis of the ABCDEFG Ring Skeleton
Abstract
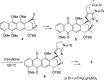
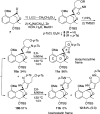
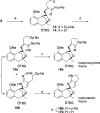
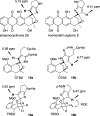
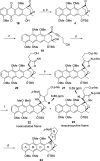
A synthetic method for the CDEFG substructure of kosinostatin was developed using the cyclization of the di-para-nosyl 3-(2-hydroxyethyl)pyrrolidin-2-imine derivative. The key to the success of this cyclization was the choice of the para-nosyloxy (p-NsO) group as the leaving group. This selection enabled the cyclization to proceed under mild reaction conditions, leading to the first construction of a kosinostatin-type scaffold. The method was further applied to the synthesis of the ABCDEFG ring of kosinostatin aglycon.
Figures

References
-
- Martin, J. H. ; Shay, A. J. ; Pruess, L. M. ; Porter, J. N. ; Mowat, J. H. ; Bohonos, N. . Paper-chromatographic studies of antibiotics produced by a Streptomyces aureofaciens. Antibiot. Annu. 1954, 1020.
- Celmer W. D., Murai K., Rao K. V., Tanner F. W. Jr., Marsh W. S.. The quinocycline complex. I. Isolation and characterization. Antibiot. Annu. 1957;5:484. - PubMed
- McBride T. J., English A. R.. The quinocycline complex. II. Biological studies. Antibiot. Annu. 1957;5:493. - PubMed
- Cosulich D. B., Mowat J. H., Broschard R. W., Patrick J. B., Meyer W. E.. Partial structure of the antibiotic isoquinocycline B. Tetrahedron Lett. 1963;4:453–458. doi: 10.1016/S0040-4039(01)90655-0. - DOI
-
Erratum Tetrahedron. Lett. 1964 750
- Tulinsky A.. The Structure of Isoquinocycline A. An X-Ray Crystallographic Determination. J. Am. Chem. Soc. 1964;86:5368. doi: 10.1021/ja01077a089. - DOI
-
- Furumai T., Igarashi Y., Higuchi H., Saito N., Oki T.. Kosinostatin, a quinocycline antibiotic with antitumor activity from Micromonospora sp. TP-A0468. J. Antibiot. 2002;55:128. doi: 10.7164/antibiotics.55.128. - DOI - PubMed
- Igarashi Y., Higuchi H., Oki T., Furumai T.. NMR analysis of quinocycline antibiotics: structure determination of kosinostatin, an antitumor substance from Micromonospora sp. TP-A0468. J. Antibiot. 2002;55:134. doi: 10.7164/antibiotics.55.134. - DOI - PubMed
- El-Naggar M. Y.. Kosinostatin, a major secondary metabolite isolated from the culture filtrate of Streptomyces violaceusniger strain HAL64. J. Microbiol. 2007;45:262. - PubMed
-
- Cordes J., Harms K., Koert U.. Synthesis of the isoquinocycline-pyrrolopyrrole substructure. Org. Lett. 2010;12:3808. doi: 10.1021/ol101500k. - DOI - PubMed
- Breuning M. A., Harms K., Koert U.. The imidato-alkenyllithium route for the synthesis of the isoquinocycline-pyrrolopyrrole substructure. Org. Lett. 2011;13:1402. doi: 10.1021/ol200085g. - DOI - PubMed
- Dischmann M., Frassetto T., Breuning M. A., Koert U.. Total synthesis of isoquinocyclinone. Chem.Eur. J. 2014;20:11300. doi: 10.1002/chem.201403880. - DOI - PubMed
- Kitamura M., Kubo K., Yoshinaga S., Matsuzaki H., Ezaki K., Matsuura T., Matsuura D., Fukuzumi N., Araki K., Narasaki M.. Synthetic study of kosinostatin aglycone: synthesis of BCDE rings using alkoxycarbonylmethylation of diazonaphthoquinone. Tetrahedron Lett. 2014;55:1653. doi: 10.1016/j.tetlet.2014.01.082. - DOI
- Kitamura M., Fukuzumi N., Shinagawa M., Araki K., Shimizu Y., Takahashi S., Iwata T., Shimooka H., Okauchi T.. Formal synthesis of isoquinocyclinone using ortho-alkoxycarbonylmethylation of anthranol via diazoquinone. Tetrahedron Lett. 2023;125:154618. doi: 10.1016/j.tetlet.2023.154618. - DOI
-
- Wuts, P. G. Greene’s Protective Groups in Organic Synthesis, 5th Ed.; John Wiley & Sons, 2014.
LinkOut - more resources
Full Text Sources

