Combinatorial discovery of microtopographical landscapes that resist biofilm formation through quorum sensing mediated autolubrication
- PMID: 40533450
- PMCID: PMC12177056
- DOI: 10.1038/s41467-025-60567-x
Combinatorial discovery of microtopographical landscapes that resist biofilm formation through quorum sensing mediated autolubrication
Abstract
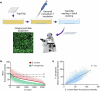
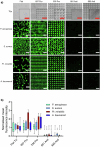
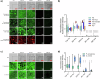
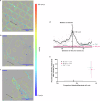
Bio-instructive materials that intrinsically inhibit biofilm formation have significant anti-biofouling potential in industrial and healthcare settings. Since bacterial surface attachment is sensitive to surface topography, we experimentally surveyed 2176 combinatorially generated shapes embossed into polymers using an unbiased screen. This identified microtopographies that, in vitro, reduce colonization by pathogens associated with medical device-related infections by up to 15-fold compared to a flat polymer surface. Machine learning provided design rules, based on generalisable descriptors, for predicting biofilm-resistant microtopographies. On tracking single bacterial cells we observed that the motile behaviour of Pseudomonas aeruginosa is markedly different on anti-attachment microtopographies compared with pro-attachment or flat surfaces. Inactivation of Rhl-dependent quorum sensing in P. aeruginosa through deletion of rhlI or rhlR restored biofilm formation on the anti-attachment topographies due to the loss of rhamnolipid biosurfactant production. Exogenous provision of N-butanoyl-homoserine lactone to the rhlI mutant inhibited biofilm formation, as did genetic complementation of the rhlI, rhlR or rhlA mutants. These data are consistent with confinement-induced anti-adhesive rhamnolipid biosurfactant 'autolubrication'. In a murine foreign body infection model, anti-attachment topographies are refractory to P. aeruginosa colonization. Our findings highlight the potential of simple topographical patterning of implanted medical devices for preventing biofilm associated infections.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


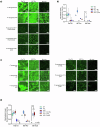
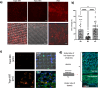
References
-
- Flemming, H. C. et al. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol.14, 563–575 (2016). - PubMed
-
- Tolker-Nielsen, T. Biofilm development. Microbiol. Spectr.3, MB-0001–MB-2014 (2015). - PubMed
-
- Laventie, B. J. & Jenal, U. Surface sensing and adaptation in bacteria. Annu. Rev. Microbiol.74, 735–760 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- EP/P029868/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- BB/R012415/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- EP/X001156/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
- 103884/WT_/Wellcome Trust/United Kingdom
- EP/N006615/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
LinkOut - more resources
Full Text Sources

