Imaging advances to detect non-motor prodromal markers of Parkinson's disease and explore therapeutic translation opportunities
- PMID: 40533452
- PMCID: PMC12177059
- DOI: 10.1038/s41531-025-01004-0
Imaging advances to detect non-motor prodromal markers of Parkinson's disease and explore therapeutic translation opportunities
Abstract
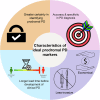
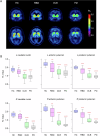
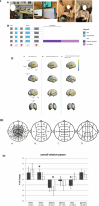
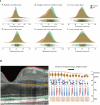
Parkinson's disease (PD) is a progressive neurological disorder marked by late-emerging motor symptoms, but early non-motor signs like hyposmia and REM sleep behavior disorder may precede diagnosis by years. Identifying non-motor biomarkers during this prodromal phase could predict phenoconversion and enable early interventions. This narrative review outlines key prodromal non-motor symptoms, summarizes imaging technologies for early detection, and explores their translational potential in guiding timely, neuroprotective therapies for at-risk individuals.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








Similar articles
-
Use of β-adrenoreceptor drugs and Parkinson's disease incidence in women from the French E3N cohort study.J Parkinsons Dis. 2025 Jun;15(4):789-804. doi: 10.1177/1877718X251330993. Epub 2025 Apr 29. J Parkinsons Dis. 2025. PMID: 40302366
-
Safety and tolerability of intravenous liposomal GM1 in patients with Parkinson disease: A single-center open-label clinical phase I trial (NEON trial).PLoS Med. 2025 May 13;22(5):e1004472. doi: 10.1371/journal.pmed.1004472. eCollection 2025 May. PLoS Med. 2025. PMID: 40359409 Free PMC article. Clinical Trial.
-
Enhancing the diagnostic potential of electroretinography in Parkinson's disease: A review of protocol and cohort criteria.J Parkinsons Dis. 2025 Jun;15(4):694-709. doi: 10.1177/1877718X251331863. Epub 2025 Apr 29. J Parkinsons Dis. 2025. PMID: 40530583 Review.
-
Incidence of antiepileptic drug use in Parkinson's disease.J Parkinsons Dis. 2025 Jun;15(4):780-788. doi: 10.1177/1877718X251343079. Epub 2025 May 23. J Parkinsons Dis. 2025. PMID: 40405650
-
Opportunities and Pitfalls of REM Sleep Behavior Disorder and Olfactory Dysfunction as Early Markers in Parkinson's Disease.J Parkinsons Dis. 2024;14(s2):S275-S285. doi: 10.3233/JPD-230348. J Parkinsons Dis. 2024. PMID: 38517805 Free PMC article. Review.
References
-
- Golan, H., Volkov, O. & Shalom, E. Nuclear imaging in Parkinson’s disease: the past, the present, and the future. J. Neurol. Sci.436, 120220 (2022). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

