Transferability of European-derived Alzheimer's disease polygenic risk scores across multiancestry populations
- PMID: 40533518
- PMCID: PMC12283339
- DOI: 10.1038/s41588-025-02227-w
Transferability of European-derived Alzheimer's disease polygenic risk scores across multiancestry populations
Abstract
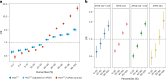
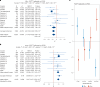
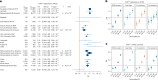
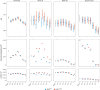
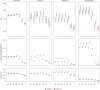
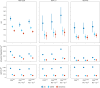
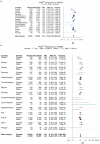
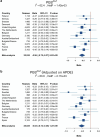
A polygenic score (PGS) for Alzheimer's disease (AD) was derived recently from data on genome-wide significant loci in European ancestry populations. We applied this PGS to populations in 17 European countries and observed a consistent association with the AD risk, age at onset and cerebrospinal fluid levels of AD biomarkers, independently of apolipoprotein E locus (APOE). This PGS was also associated with the AD risk in many other populations of diverse ancestries. A cross-ancestry polygenic risk score improved the association with the AD risk in most of the multiancestry populations tested when the APOE region was included. Finally, we found that the PGS/polygenic risk score captured AD-specific information because the association weakened as the diagnosis was broadened. In conclusion, a simple PGS captures the AD-specific genetic information that is common to populations of different ancestries, although studies of more diverse populations are still needed to better characterize the genetics of AD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.v.d.L. is a recipient of funding from ABOARD, which is a public–private partnership financed by ZonMW (no. 73305095007) and Health–Holland, Topsector Life Sciences & Health (PPP-allowance; no. LSHM20106). C.C. has received research support from GSK and EISAI. The study’s funders had no role in the collection, analysis or interpretation of data; in the writing of the report or in the decision to submit the paper for publication. C.C. is an advisory board member for Vivid Genomics and Circular Genomics and owns stock. L.M.-P. received personal fees from Biogen for consulting activities unrelated to the submitted work. T.G. received consulting fees from AbbVie, Alector, Anavex, Biogen, Cogthera, Eli Lilly, Functional Neuromodulation, Grifols, Iqvia, Janssen, Noselab, Novo Nordisk, NuiCare, Orphanzyme, Roche Diagnostics, Roche Pharma, UCB and Vivoryon; lecture fees from Biogen, Eisai, Grifols, Medical Tribune, Novo Nordisk, Roche Pharma, Schwabe and Synlab; and has received grants to his institution from Biogen, Eisai and Roche Diagnostics. O.A.A. is a consultant to Cortechs and Precision Health AS, and has received speaker’s honoraria from Lundbeck, Sunovion, Otsuka and Janssen. The other authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

