Morphodynamics of human early brain organoid development
- PMID: 40533563
- PMCID: PMC12390842
- DOI: 10.1038/s41586-025-09151-3
Morphodynamics of human early brain organoid development
Abstract
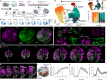
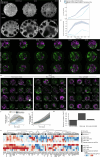
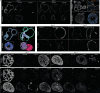
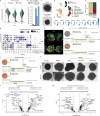
Brain organoids enable the mechanistic study of human brain development and provide opportunities to explore self-organization in unconstrained developmental systems1-3. Here we establish long-term, live light-sheet microscopy on unguided brain organoids generated from fluorescently labelled human induced pluripotent stem cells, which enables tracking of tissue morphology, cell behaviours and subcellular features over weeks of organoid development4. We provide a novel dual-channel, multi-mosaic and multi-protein labelling strategy combined with a computational demultiplexing approach to enable simultaneous quantification of distinct subcellular features during organoid development. We track actin, tubulin, plasma membrane, nucleus and nuclear envelope dynamics, and quantify cell morphometric and alignment changes during tissue-state transitions including neuroepithelial induction, maturation, lumenization and brain regionalization. On the basis of imaging and single-cell transcriptome modalities, we find that lumenal expansion and cell morphotype composition within the developing neuroepithelium are associated with modulation of gene expression programs involving extracellular matrix pathway regulators and mechanosensing. We show that an extrinsically provided matrix enhances lumen expansion as well as telencephalon formation, and unguided organoids grown in the absence of an extrinsic matrix have altered morphologies with increased neural crest and caudalized tissue identity. Matrix-induced regional guidance and lumen morphogenesis are linked to the WNT and Hippo (YAP1) signalling pathways, including spatially restricted induction of the WNT ligand secretion mediator (WLS) that marks the earliest emergence of non-telencephalic brain regions. Together, our work provides an inroad into studying human brain morphodynamics and supports a view that matrix-linked mechanosensing dynamics have a central role during brain regionalization.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures













References
-
- Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell3, 519–532 (2008). - PubMed
-
- Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J. & Stelzer, E. H. K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science305, 1007–1009 (2004). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

