Polypharmacy and sarcopenia in patients on hemodialysis: results from the SARC-HD study
- PMID: 40533714
- PMCID: PMC12176980
- DOI: 10.1007/s40520-025-03101-9
Polypharmacy and sarcopenia in patients on hemodialysis: results from the SARC-HD study
Abstract
Background: We investigated the association between polypharmacy and sarcopenia in patients on hemodialysis.
Methods: Cross-sectional data from the SARC-HD study were analyzed. Patients were classified according to the number of prescribed medications as no polypharmacy (0-4), polypharmacy (5-9), and hyperpolypharmacy (≥ 10). Sarcopenia was diagnosed and staged according to the adapted EWGSOP2 consensus.
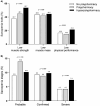
Results: 955 patients (48% ≥ 60 years, 61% male) were analyzed. Polypharmacy and hyperpolypharmacy were observed in 50% and 26% of patients, respectively. Patients with hyperpolypharmacy had poorer physical function compared to the no polypharmacy group. Low muscle strength was found in 45%, while sarcopenia (confirmed and severe stages) in 21% of the cohort. Patients in the polypharmacy groups had higher prevalence of low muscle strength, but similar sarcopenia rates to those in the no polypharmacy group. Only hyperpolypharmacy was independently associated with low muscle strength (64% higher adjusted odds, 95% CI 1.10-2.46), whereas no significant associations were observed with sarcopenia. Also, each addition of two medications was independently associated with 10% higher adjusted odds (95% CI 1.02-1.20) of low muscle strength.
Conclusions: In patients on hemodialysis, the number of medications and hyperpolypharmacy were independently associated with low muscle strength, but not with sarcopenia per se.
Keywords: Chronic kidney disease; Drug therapy; End-stage kidney disease; Frailty; Handgrip strength; Medication.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: The authors declare no competing interests. Ethical approval: This study was performed in accordance with the ethical standards and was ethically appraised by the Institutional Review Board of the University Center ICESP (no. 5.418.365; all other institutional review boards reviewed and agreed with the approval letter), which adheres to the Declaration of Helsinki. Informed consent: All participants provided written informed consent. Generative AI-assisted technologies: During the preparation of this work, the authors used ChatGPT in order to rephrase content previously published by our study group on the same project. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Figures
References
-
- Stevens PE, Ahmed SB, Carrero JJ, et al. (2024) KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 105:S117–314. https://linkinghub.elsevier.com/retrieve/pii/S0085253823007664 - PubMed
-
- Fraser SDS, Taal MW (2016) Multimorbidity in people with chronic kidney disease. Curr Opin Nephrol Hypertens 25:465–472 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical