Liposome-encapsulated antibiotics and biosurfactants: an effective strategy to boost biofilm eradication in cooling towers
- PMID: 40533735
- PMCID: PMC12175429
- DOI: 10.1186/s12934-025-02746-5
Liposome-encapsulated antibiotics and biosurfactants: an effective strategy to boost biofilm eradication in cooling towers
Abstract
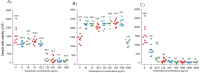
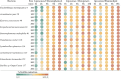
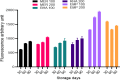
An excessive amount of water is needed for cooling towers in oil refineries to cool the machinery. However, water has been observed to favor microbial growth and biofilms significantly. The microbial biofilms are usually treated with synthetic biocides, which are ineffective and generate toxic by-products harmful to the environment. This study explores using rhamnolipid and free or encapsulated antimicrobials in liposomes to control several bacterial species exhibiting low antimicrobial susceptibility in planktonic and biofilm forms. The antimicrobial efficacy of rhamnolipid was evaluated through minimum inhibitory concentration (MIC) tests, showing values between 0.244 and 31.25 µg/mL. Biofilm inhibition assays revealed that rhamnolipid significantly reduced biofilm viability, performing comparably to meropenem and more effectively than chloramphenicol. Liposomes were produced with initial diameters of 100 and 200 nm, and encapsulation efficiencies were 56.7% for rhamnolipid, 47.3% for meropenem, and 31.25% for chloramphenicol. Among the formulations, 100 nm rhamnolipid-loaded liposomes exhibited the highest antibiofilm efficacy, achieving up to 92% biofilm reduction in Stenotrophomonas maltophilia 94 (p < 0.01). Meropenem liposomes of 100 nm also performed better than their 200 nm counterparts, with up to 85% reduction in Pseudomonas aeruginosa biofilms (p < 0.05). No significant size-dependent differences were observed for chloramphenicol liposomes, with maximum inhibition around 60% at both sizes. Long-term stability and antibiofilm activity were evaluated exclusively for S. maltophilia 94 over 90 days of refrigerated storage (4 °C). Dynamic light scattering revealed significant vesicle size increases over time for both formulations (p < 0.05), yet their antibiofilm activity remained stable. Rhamnolipid liposomes (100 nm) maintained significantly higher efficacy than 200 nm vesicles throughout the period (p < 0.01). Meropenem liposomes retained considerable activity, though a moderate decrease was noted after 60 days. Scanning electron microscopy (SEM) at days 0 and 90 confirmed the antimicrobial impact of liposomal treatments: biofilms showed disrupted architecture, reduced extracellular matrix, and evident morphological damage to bacterial cells, supporting quantitative results. These findings demonstrate that liposome-encapsulated rhamnolipids and antibiotics are effective against resilient biofilms. The successful formulation and long-term stability of rhamnolipid liposomes highlight their potential as a sustainable and eco-friendly alternative for industrial biofilm control, reducing reliance on conventional biocides and minimizing environmental impact.
Keywords: Antibiotics; Bacteria; Biofilms; Biosurfactant; Cooling towers; Liposomes; Resistance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors agreed to publish this version of the manuscript. Competing interests: The authors declare no competing interests.
Figures

References
-
- Girolamini L, Brattich E, Marino F, Rosaria M, Mazzotta M, Spiteri S et al. Cooling towers influence in an urban environment: A predictive model to control and prevent Legionella risk and Legionellosis events. Build Environ [Internet]. 2023;228:109891. Available from: 10.1016/j.buildenv.2022.109891
-
- Pippo F, Di GL, Di, Congestri R, Tandoi V, Rossetti S. Biofilm growth and control in cooling water industrial systems. FEMS Microbiol Ecol. 2018;1–13. - PubMed
-
- Proner MC, de Meneses AC, Veiga AA, Schluter H, Oliveira DD, Luccio MD. Industrial cooling systems and antibiofouling strategies: A comprehensive review. Ind Eng Chem Res. 2021;60:3278–329. - DOI
-
- Liu F, Chang X, Yang F, Wang Y. Effect of oxidizing and non-oxidizing biocides on biofilm at different substrate levels in the model recirculating cooling water system. World J Microbiol Biotechnol. 2011;2989–97.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

