Baicalin targets YTHDC2 and alleviates male reproductive toxicity caused by co-exposure to nanoplastics and manganese through m6A-dependent pathway
- PMID: 40533738
- PMCID: PMC12175420
- DOI: 10.1186/s12951-025-03535-3
Baicalin targets YTHDC2 and alleviates male reproductive toxicity caused by co-exposure to nanoplastics and manganese through m6A-dependent pathway
Abstract
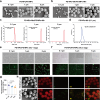
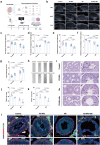
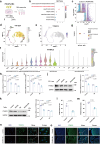
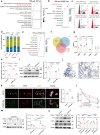
Nanoplastics (NPs) pollution has become a pressing global environmental issue. NPs possess the ability to adsorb heavy metals, thereby acting as vectors that facilitate the entry of these toxic substances into living organisms. However, the synergistic toxic effects of NPs and heavy metals, particularly with regard to male reproductive health, remain poorly understood. This study establishes in vivo and in vitro models to assess the effects of single and co-exposure to polystyrene nanoplastics (PS-NPs, 0.1 μm) and manganese (Mn) on male reproductive function. Our results reveal that co-exposure leads to a synergistic toxic effect, aggravating testicular damage, sperm abnormalities, and hormone disruption. Mechanistically, PS-NPs and Mn collaboratively suppress the RNA-binding protein YTHDC2, which in turn impairs Mdm2 transcription and translation in an m6A-dependent manner. This disruption results in cell cycle arrest via the Mdm2-p53 pathway, ultimately hindering spermatogenesis. Notably, baicalin, a natural compound, effectively targets YTHDC2 and mitigates the reproductive toxicity induced by co-exposure. These findings provide the novel evidence of the synergistic reproductive toxicity of PS-NPs and Mn in male mammals, offering new insights into their combined toxic effects and highlighting the potential of baicalin as a therapeutic intervention.
Graphical Abstract:
Supplementary Information: The online version contains supplementary material available at 10.1186/s12951-025-03535-3.
Keywords: Baicalin; Male reproductive toxicity; Manganese; Mdm2-p53 Pathway; Polystyrene nanoplastics; m6A.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This work has received approval for research ethics from the Institutional Animal Care and Use Committee of China Medical University and a proof of approval is available upon request (Approval Number: CMU2022111). Consent for publication: Consent for publication was obtained from the participants. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Protective role of m6A binding protein YTHDC2 on CCNB2 in manganese-induced spermatogenesis dysfunction.Chem Biol Interact. 2022 Jan 5;351:109754. doi: 10.1016/j.cbi.2021.109754. Epub 2021 Nov 22. Chem Biol Interact. 2022. PMID: 34822792
-
Microglial Clearance of Alzheimer's Amyloid-Beta Obstructed by Nanoplastics.Environ Sci Nano. 2025 Jun 1;12(6):3247-3260. doi: 10.1039/D5EN00291E. Epub 2025 May 6. Environ Sci Nano. 2025. PMID: 40520627
-
Repurposing MDM2 inhibitor RG7388 for TP53-mutant NSCLC: a p53-independent pyroptotic mechanism via ROS/p-p38/NOXA/caspase-3/GSDME axis.Cell Death Dis. 2025 Jun 17;16(1):452. doi: 10.1038/s41419-025-07770-2. Cell Death Dis. 2025. PMID: 40523886 Free PMC article.
-
Neuro-reproductive toxicity and carcinogenicity of 1-bromopropane: studies for evidence-based preventive medicine.J Occup Health. 2025 Jan 7;67(1):uiaf004. doi: 10.1093/joccuh/uiaf004. J Occup Health. 2025. PMID: 39869365 Free PMC article. Review.
-
Could probiotics protect against human toxicity caused by polystyrene nanoplastics and microplastics?Front Nutr. 2023 Jul 10;10:1186724. doi: 10.3389/fnut.2023.1186724. eCollection 2023. Front Nutr. 2023. PMID: 37492595 Free PMC article. Review.
Cited by
-
Ginsenoside Rb1 mitigates atherosclerosis in part through modulating FTO-mediated m6A RNA modification in NETs-induced endothelial activation.Front Pharmacol. 2025 Jul 23;16:1631076. doi: 10.3389/fphar.2025.1631076. eCollection 2025. Front Pharmacol. 2025. PMID: 40771928 Free PMC article.
References
-
- An Q, Zhou T, Wen C, Yan C. The effects of microplastics on heavy metals bioavailability in soils: a meta-analysis. J Hazard Mater. 2023;460:132369. - PubMed
-
- Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European association of urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. 2021;805:603–20. - PubMed
-
- Greeson KW, Crow KMS, Edenfield RC, Easley CA. Inheritance of paternal lifestyles and exposures through sperm DNA methylation. Nat Rev Urol. 2023;206:356–70. - PubMed
-
- Tian M, Li H, Wu S, Xi H, Wang YX, Lu YY, Wei L, Huang Q. Exposure to haloacetic acid disinfection by-products and male steroid hormones: an epidemiological and in vitro study. J Hazard Mater. 2024;468:133796. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

