Biologically targeted dual adaptive and innate nano-Immunotherapy for clear cell renal cell carcinoma treatment
- PMID: 40533745
- PMCID: PMC12175395
- DOI: 10.1186/s12943-025-02382-y
Biologically targeted dual adaptive and innate nano-Immunotherapy for clear cell renal cell carcinoma treatment
Abstract
Background: Immunotherapy treatments have significantly improved metastatic renal cell carcinoma (RCC) treatment outcomes. Despite recent advancements, the rates of durable response to immunotherapy remain low, and the toxicity profiles of treatment continue to be high. To address these challenges, we report the development of a human carbonic anhydrase-IX (hCA-9)-targeted multifunctional immunotherapy nanoparticles (MINPs) aimed at improving treatment efficacy and reducing toxicity. We hypothesized that these MINPs will facilitate the recognition and elimination of hCA-9-expressing tumor cells by both adaptive immune cells (cytotoxic CD8+ T cells) and innate immune cells (natural killer (NK) cells).
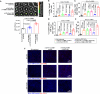
Methods: Non-targeted and hCA-9-targeted MINPs were prepared by conjugating anti-CA-9, anti-4-1BB, and anti-CD27 antibodies to poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) diblock copolymer NPs. The abilities of different MINPs in activating CD8+ T cells, NK cells, and human peripheral blood mononuclear cells (hPBMCs) were assessed. In vivo efficacy and mechanistic studies were conducted to evaluate the anticancer activities of different MINPs in immunocompetent hCA-9-transfected mouse RCC tumor models and human ccRCC xenograft models using humanized mice. We also investigated the impact of aging on anticancer efficacy of hCA-9-targeted MINPs in humanized mice. The immune-related side effects associated with the systemic administration of hCA-9-targeted MINPs were characterized.
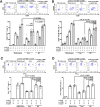
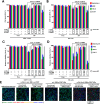
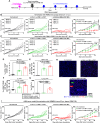
Results: Human CA-9-targeted multifunctionalized immunotherapy NPs (MINPs) functionalized with anti-CA-9, anti-4-1BB, and anti-CD27 antibodies outperformed hCA-9-targeted bifunctionalized immunotherapy NPs (BINPs), non-targeted BINPs, and the combination of free antibodies in activating mouse CD8+ T cells and NK cells to kill hCA-9-expressing RCC cells in vitro. In vivo correlative study confirmed that tumor targeting and effective spatiotemporal coactivation of the 4-1BB and CD27 pathways in CD8+ T cells and NK cells are essential for robust antitumor activity. Furthermore, hCA-9-targeted MINPs, but not the combination of free antibodies, inhibited the growth of human ccRCC in hPBMC-humanized mouse models. The anticancer activity of MINPs in mice humanized with hPBMCs from older donors was slightly weaker than in those humanized with younger donors. More importantly, the MINP formulation effectively prevented the hepatotoxicity associated with the systemic administration of immune checkpoint agonistic antibodies.
Conclusion: This study demonstrates that MINPs are a versatile platform capable of facilitating immune cell engagement and the eradication of targeted ccRCC without causing systemic immune-related side effects.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were approved by the University of Texas Southwestern Medical Center Institutional Animal Care (protocol 2023-103631) following the Guideline for the Care and Use of Laboratory Animals (NIH publication no. 86 − 23, revised 1985). Consent for publication: All authors have consented to submit this article for publication. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

