Exosomes as nanocarriers for brain-targeted delivery of therapeutic nucleic acids: advances and challenges
- PMID: 40533746
- PMCID: PMC12178025
- DOI: 10.1186/s12951-025-03528-2
Exosomes as nanocarriers for brain-targeted delivery of therapeutic nucleic acids: advances and challenges
Abstract
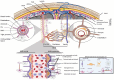
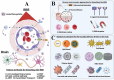
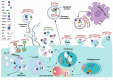
Recent advancements in gene expression modulation and RNA delivery systems have underscored the immense potential of nucleic acid-based therapies (NA-BTs) in biological research. However, the blood-brain barrier (BBB), a crucial regulatory structure that safeguards brain function, presents a significant obstacle to the delivery of drugs to glial cells and neurons. The BBB tightly regulates the movement of substances from the bloodstream into the brain, permitting only small molecules to pass through. This selective permeability poses a significant challenge for effective therapeutic delivery, especially in the case of NA-BTs. Extracellular vesicles, particularly exosomes, are recognized as valuable reservoirs of potential biomarkers and therapeutic targets. They are also gaining significant attention as innovative drug and nucleic acid delivery (NAD) carriers. Their unique ability to safeguard and transport genetic material, inherent biocompatibility, and capacity to traverse physiological barriers highlight their potential as drug carriers. This review provides a comprehensive overview of current strategies to enhance NAD to the brain, focusing on the emerging potential of exosomes as biocompatible and efficient nanocarriers. It synthesizes recent advances in the use of exosomes for NA-BTs in neurological disorders, comparing their advantages with those of conventional nanodelivery systems and cell-based therapies. Additionally, the review highlights innovative exosome engineering approaches to improve brain-targeted delivery, addresses key methodological limitations such as variability in cargo content, and proposes solutions to enhance standardization and safety. Collectively, these insights highlight the translational potential of exosomes and offer a novel perspective on bridging the gap between fundamental research and clinical application.
Keywords: BBB; Exosomes; Neurodegeneration; Neuroinflammation; Neuropharmacology.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

