Increased GPR35 expression is correlated with poor prognosis in prostate cancer
- PMID: 40533764
- PMCID: PMC12175426
- DOI: 10.1186/s12957-025-03893-0
Increased GPR35 expression is correlated with poor prognosis in prostate cancer
Abstract
Background: G-protein-coupled receptor 35 (GPR35) has been reported to be overexpressed in several types of human cancers, playing essential roles in tumorigenesis and development. However, its expression and prognostic value in Prostate cancer (PCa) remain unclear. This study aims to investigate the expression of GPR35 and its prognostic value in PCa.
Methods: The expression of GPR35 was analyzed using the public database and validated by immunohistochemistry (IHC) in PCa tissues. Subsequently, the correlation between GPR35 expression and the clinical characteristics was evaluated using the Chi-squared test. Kaplan-Meier and Cox proportional hazards regression models were used to analyze the data. Hazard Ratios (HR) and 95% confidence intervals (CI) were calculated for each factor.
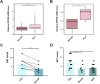
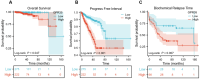
Results: GPR35 messenger RNA (mRNA) and protein expression were confirmed to be overexpressed in PCa tissue samples. Furthermore, high GPR35 mRNA expression was correlated with clinical tumor stage (T stage) (P < 0.001), lymph node metastasis (P < 0.001), primary therapy outcome (P = 0.009), residual tumor (P < 0.001), prostate-specific antigen (PSA) levels (P = 0.004), and Gleason score (P < 0.001). IHC analysis also confirmed that GPR35 overexpression was associated with lymph node metastasis (P = 0.010). Additionally, Kaplan-Meier analysis showed that PCa patients with high expression of GPR35 were associated with shorter overall survival (OS) (HR: 3.370, 95% CI: 1.085-10.470, P = 0.047), progress free interval (PFI) (HR: 3.385, 95% CI: 2.234-5.131, P < 0.001), and biochemical relapse time (BCR) (HR: 2.229, 95% CI: 1.308-3.801, P = 0.007). Moreover, univariate Cox regression analyses suggested that T stage (P < 0.001), lymph node involvement (P = 0.046), serum PSA levels (P = 0.013), Gleason score (P < 0.001), and GPR35 expression (P < 0.001) were unfavorable prognostic factors for PCA patients. Multivariate Cox regression analysis showed that GPR35 was an independent poor prognostic factor of PCa patients (HR: 1.915, 95%CI: 1.368-2.682).
Conclusion: Overexpression of GPR35 is associated with poor clinical prognosis, suggesting that GPR35 may serve as a potential prognostic biomarker for PCa.
Clinical trial number: Not applicable.
Keywords: Biomarkers; GPR35; Prognosis; Prostate; Prostate cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of the General Hospital of Xinjiang Military Region of the Chinese People’s Liberation Army and conducted in accordance with the 1996 Declaration of Helsinki. The Ethics Committee of the General Hospital of Xinjiang Military Region of the Chinese People’s Liberation Army waived informed consent from participants because this study involved routinely collected medical data that were managed anonymously at all stages, including the data cleaning and statistical analysis stages. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

