The DCDC2/ENO1 axis promotes tumor progression and immune evasion in intrahepatic cholangiocarcinoma via activating FGL1-LAG3 checkpoint
- PMID: 40533767
- PMCID: PMC12175362
- DOI: 10.1186/s13046-025-03436-1
The DCDC2/ENO1 axis promotes tumor progression and immune evasion in intrahepatic cholangiocarcinoma via activating FGL1-LAG3 checkpoint
Abstract
Background & aims: ICC is a malignant tumor that originates from the intrahepatic bile ducts with insidious symptoms and a poor prognosis. Early diagnosis methods and therapeutic targets are urgently needed for ICC.
Methods: We utilized a comprehensive set of analytical techniques to elucidate the role and mechanisms of DCDC2 in ICC. Our study included protein microarrays, transcriptome analysis, functional assays, immunofluorescence, dual-luciferase reporter assays, as well as xenograft models and humanized PBMC models.
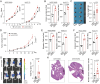
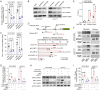
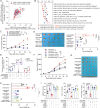
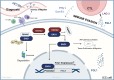
Results: Our study demonstrates that elevated levels of anti-DCDC2 autoantibodies in the serum of ICC patients indicate its potential utility as a diagnostic biomarker. Comprehensive in vitro and in vivo analyses reveal that DCDC2 promotes ICC proliferation, metastasis, and immune evasion. Mechanistically, DCDC2 stabilizes ENO1, resulting in enhanced AKT phosphorylation and increased expression of FGL1. Notably, elevated FGL1 levels significantly impair CD8+ T cell functionality via the FGL1-LAG3 axis.
Conclusion: Our findings position anti-DCDC2 autoantibody as a promising diagnostic biomarker for ICC, associated with poor prognostic outcomes, and elucidate its critical role in tumor growth and immune evasion through its interaction with ENO1.
Keywords: Doublecortin domain containing 2; Enolase 1; Fibrinogen-like protein 1; Immune evasion; Intrahepatic Cholangiocarcinoma; Progression; Tumor-associated antigen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal research was approved by the Institutional Animal Care and Use Committee of Shanghai Immunocan Biotechnology Co., Ltd (Approval Number: YMNK-AUA-01201, Approval Date: February 17th, 2023). The human research was approved by the Clinical Research Ethics Committee of Fudan University Zhongshan Hospital (Approval Number: B2023-299R2, Approval Date: December 11, 2023). Additionally, it should be noted that at our teaching hospital, all patients admitted for surgical procedures are required to sign consent forms allowing for the use of their surgical specimens for research purposes. Therefore, research involving surgical specimens is exempt from the requirement for explicit informed consent. Consent for publication: All authors have agreed with publishing this manuscript. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- 82272772, 81872352, 82072682, 82173106, 82130115/National Natural Science Foundation of China
- 82272772, 81872352, 82072682, 82173106, 82130115/National Natural Science Foundation of China
- 82272772, 81872352, 82072682, 82173106, 82130115/National Natural Science Foundation of China
- 21ZR1459100/Natural Science Foundation of Shanghai
- 21ZR1459100/Natural Science Foundation of Shanghai
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

